Documentos de Académico
Documentos de Profesional
Documentos de Cultura
UNIFAC 3componentes
Cargado por
Amner Rudhy Fonseca TelloDescripción original:
Título original
Derechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
UNIFAC 3componentes
Cargado por
Amner Rudhy Fonseca TelloCopyright:
Formatos disponibles
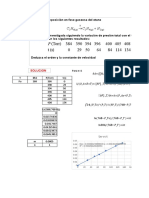
CALCULOS EN UNIFAC
Sistema: acetona(1)/metanol(2)/agua(3) CH3-CO-CH3/CH3OH/H2O
DATOS
CUADRO No 1. Parámetros de las sustancias Parámetros de interacción bina
NG Vol. F R Área F R CH3 (1)
vk1 vk2 vk3
k Rk Qk 1
1 (1)CH3 (1) 0.9011 0.848 1 0 0 0
2 CH3CO(9) 1.6724 1.488 1 0 0 26.76
3 CH3OH (6) 1.4311 1.432 0 1 0 16.51
4 H2O (7) 0.92 1.4 0 0 1 300
NG
Gk1 Gk2 Gk3 q1 q2 q3
k
1 CH3 0.848 0 0 0.848 0 0
2 CH3CO 1.488 0 0 1.488 0 0
3 CH3OH 0 1.432 0 0 1.432 0
4 H2O 0 0 1.4 0 0 1.4
2.336 1.432 1.4
i A B C
1 Acetona 14.3916 2795.82 230.00
2 Metanol 16.5938 3644.30 239.76
3 Agua 16.2620 3799.89 226.35
i xi t(°C) Psati P(KPa) yi
1 0.3 70 159.5249 125.54523 0.5085
2 0.4 125.07146 0.4089
3 0.3 31.177457 0.0826
1
n 1 2 3
L1 1.379 1.379 1.379
L2 0.846 0.846 0.846
L3 0.827 0.827 0.827
J1 1.458 1.458 1.458
J2 0.811 0.811 0.811
J3 0.793 0.793 0.793
θ1 0.254 0.254 0.254
θ2 0.446 0.446 0.446
θ3 0.573 0.573 0.573
θ4 0.420 0.420 0.420
t 50.00 70.000 69.981
T 323.00 343.00 342.98
s11 2.218 2.224 2.224
s21 1.682 1.699 1.699
s31 1.161 1.195 1.195
s41 0.359 0.393 0.393
s12 1.361 1.365 1.365
s22 1.332 1.338 1.338
s32 1.432 1.432 1.432
s42 2.508 2.427 2.427
s13 0.553 0.584 0.584
s23 2.564 2.475 2.475
s33 0.571 0.602 0.602
s43 1.400 1.400 1.400
η1 1.375 1.388 1.388
η2 1.806 1.787 1.787 𝜼_𝒌=∑1_𝒊▒ 〖𝒔 _(𝒌,𝒊) 𝒙_𝒊 〗
η3 1.092 1.112 1.112
η4 1.531 1.509 1.509
Ganma 1 1.3339 1.3361 1.3361
Ganma 2 1.0262 1.0231 1.0231
Ganma 3 1.1086 1.1250 1.1250
Psat1 159.52 159.40 159.40
Psat2 125.07 124.97 124.97
Psat3 31.18 31.15 31.15
t 70.00000 69.98121 69.98121
CH3-CO-CH3/CH3OH/H2O
Parámetros de interacción binaria
CH3CO(9) CH3OH(6) H2O (7)
2 3 4
476.4 697.2 1318
0 108.7 472.5
23.39 0 -181
-195.4 289.6 0
r1 r2 r3
0.9011 0 0
1.6724 0 0
0 1.4311 0
0 0 1.4
2.5735 1.4311 1.4
𝒒_𝒊=∑1_𝒌▒𝑮_(𝒌,𝒊)
𝑟_𝑖=∑1_𝑘▒ 〖
𝜈 _(𝑘,𝑖) 𝑅_𝑘 〗
𝑳_𝒊=𝒒_𝒊/(∑1_𝒋▒ 〖𝒒 _𝒋 𝒙_𝒋 〗 )
𝒋_𝒊=𝒓_𝒊/(∑1_𝒋▒ 〖𝒓 _𝒋 𝒙_𝒋 〗 )
𝜽_𝒌=∑1_𝒊▒ 〖𝑮 _(𝒌,𝒊) 𝒙_𝒊 〗
𝜞_(𝒌,𝒍)=𝒆𝒙𝒑((−𝜶_(𝒌,𝒍))/𝑻)
𝒔_(𝒌,𝒊)=∑1_𝒍▒ 〖𝑮 _(𝒍,𝒊) 𝜞_(𝒍,𝒌) 〗
〖𝑙𝑛𝛾 〗_1^𝑅=𝑞_1 (1− 〖 𝑙𝑛𝐿〗_1 )−[(𝜃_1 𝑠_1,1/𝜂_1 +𝜃_2 𝑠_2,1/𝜂_2 +𝜃_3 𝑠_3,1/𝜂_3 +𝜃_4 𝑠_4,1/𝜂_4 )−(𝐺_1,1
+𝐺_2,1 𝑙𝑛 𝑠_2,1/𝜂_2 +𝐺_3,1 𝑙𝑛 𝑠_3,1/𝜂_3 +𝐺_4,1 𝑙𝑛 𝑠_4,1/𝜂_4 )]
〖
𝑙𝑛𝛾 〗_2^𝑅=𝑞_2 (1− 〖
𝑙𝑛𝐿〗_2 )−[(𝜃_1 𝑠_1,2/𝜂_1 +𝜃_2 𝑠_2,2/𝜂_2 +𝜃_3 𝑠_3,2/𝜂_3 +𝜃_4 𝑠_4,2/𝜂_4 )−(𝐺_1,
_𝒊▒ 〖𝒔 _(𝒌,𝒊) 𝒙_𝒊 〗𝑙𝑛 𝑠_2,2/𝜂_2 +𝐺_3,2 𝑙𝑛 𝑠_3,2/𝜂_3 +𝐺_4,2 𝑙𝑛 𝑠_4,2/𝜂_4 )]
〖𝑙𝑛𝛾 〗_3^𝑅=𝑞_3 (1− 〖 𝑙𝑛𝐿〗_3 )−[(𝜃_1 𝑠_1,3/𝜂_1 +𝜃_2 𝑠_2,3/𝜂_2 +𝜃_3 𝑠_3,3/𝜂_3 +𝜃_4 𝑠_4,3/𝜂_4 )−(𝐺_1
𝑠_2,3/𝜂_2 +𝐺_3,3 𝑙𝑛 𝑠_3,3/𝜂_3 +𝐺_4,3 𝑙𝑛 𝑠_4,3/𝜂_4 )]
〖 lnγ 〗 _i^C=1−J_i+lnJ_i−5q_i (1−J_i/L_i +ln J_i/L_i )
𝑟_𝑖=∑1_𝑘▒ 〖
𝜈 _(𝑘,𝑖) 𝑅_𝑘 〗
𝜂_3 +𝜃_4 𝑠_4,1/𝜂_4 )−(𝐺_1,1 𝑙𝑛 𝑠_1,1/𝜂_1
/𝜂_3 +𝜃_4 𝑠_4,2/𝜂_4 )−(𝐺_1,2 𝑙𝑛 𝑠_1,2/𝜂_1 +𝐺_2,2
/𝜂_3 +𝜃_4 𝑠_4,3/𝜂_4 )−(𝐺_1,3 𝑙𝑛 𝑠_1,3/𝜂_1 +𝐺_2,3 𝑙𝑛
También podría gustarte
- Problemas resueltos de Hidráulica de CanalesDe EverandProblemas resueltos de Hidráulica de CanalesCalificación: 4.5 de 5 estrellas4.5/5 (7)
- Problemas Propuestos 01 2022-0 - Mora Guevara FlaviaDocumento24 páginasProblemas Propuestos 01 2022-0 - Mora Guevara FlaviaNarda RamosAún no hay calificaciones
- Tarea 2 ELVDocumento18 páginasTarea 2 ELVAmy GuillenAún no hay calificaciones
- Indice de RefraccionDocumento6 páginasIndice de Refraccionroman pintorAún no hay calificaciones
- Unifac TablaDocumento9 páginasUnifac TablaANDERSSONAún no hay calificaciones
- Protocolo Trafo 37.5 Kva - MagnetronDocumento2 páginasProtocolo Trafo 37.5 Kva - MagnetronAlejandro Castro Gomez100% (2)
- UNIFAC Etanol-AguaDocumento5 páginasUNIFAC Etanol-AguaRomel Casafranca HuallparimachiAún no hay calificaciones
- Cálculos de PropiedadesDocumento107 páginasCálculos de PropiedadesHsiullAún no hay calificaciones
- Practica 6Documento16 páginasPractica 6ruddy sniderAún no hay calificaciones
- Unifac Etanol-Agua ErrorDocumento5 páginasUnifac Etanol-Agua ErrorVictor Alca SalcedoAún no hay calificaciones
- Coef Fugacidad TernarioDocumento16 páginasCoef Fugacidad TernarioJohn CAún no hay calificaciones
- Diagramas de HeislerDocumento7 páginasDiagramas de HeislerLaura Cubillos RodríguezAún no hay calificaciones
- Cálculos de Propiedades - Valentina Hernández, Carlos GerdezDocumento232 páginasCálculos de Propiedades - Valentina Hernández, Carlos GerdezHsiullAún no hay calificaciones
- Práctica 10Documento14 páginasPráctica 10Joshua cervantes enriquezAún no hay calificaciones
- Tarea de CBF 211l Pract. 01 (Pendulo Fisico) (Ismael 1096414)Documento9 páginasTarea de CBF 211l Pract. 01 (Pendulo Fisico) (Ismael 1096414)Ismael Bencosme TapiaAún no hay calificaciones
- Tarea Practica 1 PENDULODocumento15 páginasTarea Practica 1 PENDULOGian SusanaAún no hay calificaciones
- BIOTECNOLOGIADocumento11 páginasBIOTECNOLOGIAAgustina VerdeAún no hay calificaciones
- Borrador Diseño de Diametro Marcop 260522Documento108 páginasBorrador Diseño de Diametro Marcop 260522Marco Gonzalez GarciaAún no hay calificaciones
- GRAFICAS MruaDocumento3 páginasGRAFICAS Mruabranviruzbonilla12Aún no hay calificaciones
- Acetona (1) N-PentanoDocumento5 páginasAcetona (1) N-PentanoRaque PcAún no hay calificaciones
- Copia de Grupo B - DifusiónDocumento21 páginasCopia de Grupo B - DifusiónBRISA ROJAS GUTIERREZAún no hay calificaciones
- CASOS PRACTICOS RefrigeracionDocumento15 páginasCASOS PRACTICOS Refrigeracioning_marin69Aún no hay calificaciones
- BLOQUESDocumento9 páginasBLOQUESHuesinho HRAún no hay calificaciones
- Teves 24 de SetDocumento10 páginasTeves 24 de SetJunior EncaladaAún no hay calificaciones
- Practica 1 Lab Fisica 2Documento7 páginasPractica 1 Lab Fisica 2LFAún no hay calificaciones
- Tarea XI TermoDocumento42 páginasTarea XI TermoGabriel Arturo Castillo CcorimayyaAún no hay calificaciones
- Trabajo Final GAC (TCE)Documento15 páginasTrabajo Final GAC (TCE)rolando lainezAún no hay calificaciones
- Distribución de Tiempos de Residencia en Reactores de Flujo No IdealDocumento10 páginasDistribución de Tiempos de Residencia en Reactores de Flujo No IdealClaudia GonzalesAún no hay calificaciones
- TAREA DE CBF 211L Pract. 01 (Pendulo Fisico)Documento7 páginasTAREA DE CBF 211L Pract. 01 (Pendulo Fisico)Gregory HerreraAún no hay calificaciones
- Movimiento CircularDocumento6 páginasMovimiento CircularUlises Fabian Hernandez Torres0% (1)
- Parámetros Cromatográficos en CG Datos ExperimentalesDocumento8 páginasParámetros Cromatográficos en CG Datos ExperimentalesPeña GutierrezAún no hay calificaciones
- Simulador TareaDocumento5 páginasSimulador TareaFernanda DomínguezAún no hay calificaciones
- Informe N°2 (Mezclas Azeótropicas) Grupo 4Documento19 páginasInforme N°2 (Mezclas Azeótropicas) Grupo 4Frank BustosAún no hay calificaciones
- Reporte L-4 LTPDocumento9 páginasReporte L-4 LTPOscar Alejandro Trujillo GutiérrezAún no hay calificaciones
- Tarea para 02 JunioDocumento8 páginasTarea para 02 JunioGabriel Monzòn LunaAún no hay calificaciones
- Segundo Parcial Reservorios IiDocumento10 páginasSegundo Parcial Reservorios IiMiguel MartinezAún no hay calificaciones
- Ejercicio 19.1 Escobedo ArantxaDocumento4 páginasEjercicio 19.1 Escobedo ArantxaLucas Escudero RamírezAún no hay calificaciones
- Friccion Dinamica CálculosDocumento9 páginasFriccion Dinamica CálculosViridiana RojasAún no hay calificaciones
- Tabla 1 MCUVDocumento2 páginasTabla 1 MCUVJerry QuishpeAún no hay calificaciones
- Perdida de CargaDocumento9 páginasPerdida de CargaEfrain Edgar Meneses MalcaAún no hay calificaciones
- Perdida de CargaDocumento9 páginasPerdida de CargaAndrés ErickAún no hay calificaciones
- Vant HoofDocumento6 páginasVant HoofAle FloresAún no hay calificaciones
- ENTREGA 2, CAMPO LLANITOfDocumento13 páginasENTREGA 2, CAMPO LLANITOfNicolasToroAriasAún no hay calificaciones
- Práctica4 Termo EqDocumento9 páginasPráctica4 Termo EqDafne Itzel Victorio LanderosAún no hay calificaciones
- Diagrama de Equilibrio Liquido - Vapor para El Sistema N-Pentano y N-Hexano - Lizbeth Gonzalez HernandezDocumento4 páginasDiagrama de Equilibrio Liquido - Vapor para El Sistema N-Pentano y N-Hexano - Lizbeth Gonzalez HernandezLizbeth Gonzalez HernandezAún no hay calificaciones
- Ley de Raoult Binario y TernarioDocumento10 páginasLey de Raoult Binario y TernarioPatriciaLlacsaLázaroAún no hay calificaciones
- Cilindros ConcentricosDocumento8 páginasCilindros ConcentricosAndrea CerveraAún no hay calificaciones
- Ejercicio Examen COMPLETODocumento14 páginasEjercicio Examen COMPLETOKaren LegorretaAún no hay calificaciones
- Hidrologia MuñizDocumento18 páginasHidrologia MuñizBryan KenaiAún no hay calificaciones
- TAller #2 DinaDocumento16 páginasTAller #2 DinaPaula Andrea Escorcia AhumadaAún no hay calificaciones
- Examen Unidad 2Documento8 páginasExamen Unidad 2Rodrigo CuellarAún no hay calificaciones
- Alan Alberto Aragón Landeros-Examen Sistemas TermicosDocumento10 páginasAlan Alberto Aragón Landeros-Examen Sistemas TermicosJeniferAún no hay calificaciones
- Cuestionario 3Documento4 páginasCuestionario 3grupoaepuAún no hay calificaciones
- UTN FRH TP N2 - Ventiladores - Turbomáquinas - Grupo 1Documento7 páginasUTN FRH TP N2 - Ventiladores - Turbomáquinas - Grupo 1Malen MartinezAún no hay calificaciones
- EntalpiaVap ClF3Documento3 páginasEntalpiaVap ClF3Emanuel MenesesAún no hay calificaciones
- Práctica 1 Hidrodinámica de Reactores Airlift-LoopDocumento8 páginasPráctica 1 Hidrodinámica de Reactores Airlift-LoopGABEEBBAún no hay calificaciones
- Excel de Laboratorio 07 de Física IIDocumento4 páginasExcel de Laboratorio 07 de Física IIAdriana Tena BerrocalAún no hay calificaciones
- Examen Final-Fq-2004-IDocumento4 páginasExamen Final-Fq-2004-IwefweFWEfAún no hay calificaciones
- Task2 IrqDocumento9 páginasTask2 IrqSr. CosmosAún no hay calificaciones
- Tarea Diseño de PavimentosDocumento5 páginasTarea Diseño de PavimentosJose LopezAún no hay calificaciones
- Valdivia - Informe 5 - "Valoraciones Conductimétricas"Documento26 páginasValdivia - Informe 5 - "Valoraciones Conductimétricas"Amner Rudhy Fonseca TelloAún no hay calificaciones
- Informe N°1-Labo Analisis Intrumental-Normas de Seguridad en El LaboratorioDocumento2 páginasInforme N°1-Labo Analisis Intrumental-Normas de Seguridad en El LaboratorioAmner Rudhy Fonseca TelloAún no hay calificaciones
- 91g Informes 7-8 Laboratorio Semana 4Documento23 páginas91g Informes 7-8 Laboratorio Semana 4Amner Rudhy Fonseca TelloAún no hay calificaciones
- INFORME N°1-Microbiologia 91GDocumento29 páginasINFORME N°1-Microbiologia 91GAmner Rudhy Fonseca TelloAún no hay calificaciones
- Informe 2 Tinción Gram GRUPO 1Documento16 páginasInforme 2 Tinción Gram GRUPO 1Amner Rudhy Fonseca TelloAún no hay calificaciones
- Informe 8-Organica-IIDocumento29 páginasInforme 8-Organica-IIAmner Rudhy Fonseca TelloAún no hay calificaciones
- Programming GE CL3 Universal Remote CodesDocumento35 páginasProgramming GE CL3 Universal Remote Codesh99vd9h492Aún no hay calificaciones
- Guía de Trabajo Del Estudiante Electricidad Básica, Dispositivos EléctricosDocumento8 páginasGuía de Trabajo Del Estudiante Electricidad Básica, Dispositivos EléctricosCristian Luengo BaezaAún no hay calificaciones
- Manual Curso SaisDocumento52 páginasManual Curso SaisPAULI PULAún no hay calificaciones
- LABORATORIO N 4 Elt 2641Documento10 páginasLABORATORIO N 4 Elt 2641Milton Guzman MenciaAún no hay calificaciones
- Diagrama de Bode (Tablas)Documento1 páginaDiagrama de Bode (Tablas)Juan Manuel BalderramaAún no hay calificaciones
- Catalogo Electronica ShopDocumento110 páginasCatalogo Electronica ShopaeduardocgAún no hay calificaciones
- Actividad 4 Planeacion y Control de La CalidadDocumento4 páginasActividad 4 Planeacion y Control de La CalidadTomas Uriel Dominguez Cespedes100% (2)
- Interfaces - OperacionalesDocumento11 páginasInterfaces - OperacionalesFranco RibaAún no hay calificaciones
- Inversor de 25W (Gama Baja)Documento4 páginasInversor de 25W (Gama Baja)Tarcisio MacedoAún no hay calificaciones
- Inversor de Puente CompletoDocumento7 páginasInversor de Puente CompletoDaniel Cano SuarezAún no hay calificaciones
- Guia #5 - de - Aprendizaje EncendidoDocumento13 páginasGuia #5 - de - Aprendizaje EncendidoLuis Anibal Velasquez CeballosAún no hay calificaciones
- Rúbrica OMINDocumento7 páginasRúbrica OMINJOSE GILBERTO CANO GREENEAún no hay calificaciones
- Grupo10 Electrónica Lab2Documento13 páginasGrupo10 Electrónica Lab2Equals Inspire VegaAún no hay calificaciones
- Diseño de Sistemas DigitalesDocumento10 páginasDiseño de Sistemas DigitalesАдриан БандаAún no hay calificaciones
- Multimetro y Amperimetro de GanchoDocumento11 páginasMultimetro y Amperimetro de GanchoYazmin RodriguezAún no hay calificaciones
- Análisis Del Flip-Flop Rs. Aplicaciones Del Flip-Flop JK - Parado Sosa Daniel ElmerDocumento21 páginasAnálisis Del Flip-Flop Rs. Aplicaciones Del Flip-Flop JK - Parado Sosa Daniel ElmerDaniel Elmer Parado SosaAún no hay calificaciones
- Procedimiento Resistencia VariableDocumento3 páginasProcedimiento Resistencia VariableJohnny AybarAún no hay calificaciones
- Tarea2 CIA ErazoDuranJoseLuisDocumento15 páginasTarea2 CIA ErazoDuranJoseLuisJose Luis Erazo DuranAún no hay calificaciones
- Guía de Práctica N12 Contadores IntegradosDocumento7 páginasGuía de Práctica N12 Contadores IntegradosYODY YSIDRO LOZANO SUAREZAún no hay calificaciones
- ApuntesDocumento17 páginasApuntesDiana VillegasAún no hay calificaciones
- ETD PrEPARATORIO 1 22 23Documento4 páginasETD PrEPARATORIO 1 22 23Joel QuilcaAún no hay calificaciones
- C. Analisis de Corto Circuito y Falla A Tierra OkDocumento2 páginasC. Analisis de Corto Circuito y Falla A Tierra OkJorge100% (1)
- ProyPROYECTO DE UN SISTEMA DE MEGAFONIA PARA UN CIRCUITO DE CARRERAS DE VELOCIDADecto de Un Sistema de Megafonia para Un Circuito de Carreras de VelocidadDocumento5 páginasProyPROYECTO DE UN SISTEMA DE MEGAFONIA PARA UN CIRCUITO DE CARRERAS DE VELOCIDADecto de Un Sistema de Megafonia para Un Circuito de Carreras de VelocidadorebuAún no hay calificaciones
- Trabajo de PanelesDocumento23 páginasTrabajo de PaneleshugoAún no hay calificaciones
- ProteusDocumento12 páginasProteusGabriela Mayorga SanchezAún no hay calificaciones
- Multivibradores y Sus TiposDocumento7 páginasMultivibradores y Sus TiposdargeliAún no hay calificaciones
- PRESUDocumento8 páginasPRESUNicolas CastroAún no hay calificaciones
- Prácticas V2 de Laboratorio de Electrónica Aplicada 2019Documento4 páginasPrácticas V2 de Laboratorio de Electrónica Aplicada 2019Marco Antonio Solis OrtegaAún no hay calificaciones