Documentos de Académico
Documentos de Profesional
Documentos de Cultura
OZONE
Cargado por
JITENDER PAL SINGHDescripción original:
Derechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
OZONE
Cargado por
JITENDER PAL SINGHCopyright:
Formatos disponibles
Ozone
IUPAC name[hide]Trioxygen Identifiers CAS number 10028-15-6 Y PubChem 24823 ChemSpider 23208 Y UNII 66H7ZZK23N Y EC number 2330692 MeSH Ozone ChEBI CHEBI:25812 Y RTECS number RS8225000 Gmelin Reference 1101 Jmol-3D images Image 1 Image 2 SMILES [show]o:o:o -------------------------------------------------------------------------------[O]O[O]
InChI [show]InChI=1S/O3/c1-3-2 Y Key: CBENFWSGALASAD-UHFFFAOYSA-N Y -------------------------------------------------------------------------------InChI=1/O3/c1-3-2 Key: CBENFWSGALASAD-UHFFFAOYAY Properties Molecular formula O3 Molar mass 48 g mol1 Appearance Pale blue gas Density 2.144 mg cm3 (at 0 C) Melting point 192 C, 81 K, 314 F
Solubility in water 1.05 g L1 (at 0 C) Refractive index (nD) 1.2226 (liquid) Structure Space group C2v Coordination geometry Digonal Molecular shape Dihedral Hybridisation sp2 for O1 Dipole moment 0.53 D Thermochemistry Std enthalpy of formation fHo298 142.67 kJ mol1 Standard molar entropy So298 238.92 J K1 mol1 Hazards EU classification O NFPA 704 044OX
Boiling point 112 C, 161 K,
170 F
Related compounds Related compounds Sulfur dioxide Trisulfur Disulfur monoxide Cyclic ozone Y (verify) (what is: Y/N?) Except where noted otherwise, data are given for materials in their standard sta te (at 25 C, 100 kPa) Infobox references Ozone ( /o zo n/; O3), or trioxygen, is a triatomic molecule, consisting of three oxy gen atoms. It is an allotrope of oxygen that is much less stable than the diatom ic allotrope (O2), breaking down with a half life of about half an hour in the l ower atmosphere, to normal dioxygen. Ozone is formed from dioxygen by the action of ultraviolet light and also atmospheric electrical discharges, and is present in low concentrations throughout the Earth's atmosphere. In total, ozone makes up only 0.6 parts per million of the atmosphere. Ozone was proposed as a new substance in air in 1840, and named, even before its chemical nature was known, after the Greek verb ozein (, "to sm ll"), from th p cul ar odor aft r l ght g storms. O o 's odor s sharp, r m sc t of chlor , a d d t ctabl by ma y p opl at co c trat o s of as l ttl as 10 parts p r b ll o a r. O o 's O3 formula was d t rm d 1865. Th mol cul was lat r prov to hav a b t structur a d to b d amag t c. I sta dard co d t o s, o o s a pal blu gas that co d s s at progr ss v ly cryog c t mp ratur s t o a dark blu l qu d a d f ally a v ol t-black sol d. O o 's stab l ty w th r gard to mor commo d oxyg s such that both co c trat d gas a d l qu d o o may d compos xplos v ly. It s th r for us d comm rc ally o ly low co c trat o s.
Co t ts [h d ] 1 H story 2 Phys cal prop rt s 3 Structur 4 R act o s 4.1 W th m tals 4.2 W th trog a d carbo compou ds 4.3 W th sulfur compou ds 4.4 W th alk s a d alky s 4.5 Oth r substrat s 4.6 Combust o 4.7 R duct o to o o d s 4.8 Appl cat o s 5 O o Earth's atmosph r 5.1 O o lay r 5.1.1 Locat o a d product o 5.1.2 Importa c to surfac -dw ll g l f o Earth 5.2 Low l v l o o 5.2.1 O o crack g 5.2.2 O o as a gr hous gas
O o s a pow rful ox da t (far mor so tha d oxyg ) a d has ma y dustr al a d co sum r appl cat o s r lat d to ox d at o . Th s sam h gh ox d g pot t al, how v r, caus s o o to damag mucus a d r sp ratory t ssu s a mals, a d also t ssu s pla ts, abov co c trat o s of about 100 parts p r b ll o . Th s mak s o o a pot t r sp ratory ha ard a d polluta t ar grou d l v l. H ow v r, th so-call d o o lay r (a port o of th stratosph r w th a h gh r c o c trat o of o o , from two to ght ppm) s b f c al, pr v t g damag g ultrav ol t l ght from r ach g th Earth's surfac , to th b f t of both pla ts a d a mals.
[ d t] H storyO o , th f rst allotrop of a y ch m cal l m t to b r cog d, was propos d as a d st ct ch m cal substa c by Chr st a Fr dr ch Sch b 1840, who am d t aft r th Gr k v rb o (, "to sm ll"), from th p cul ar od or l ght g storms.[1][2] Th formula for o o , O3, was ot d t rm d u t l 1865 by Jacqu s-Lou s Sor t[3] a d co f rm d by Sch b 1867.[1][4]
Most people can detect about 0.01 mol/mol of ozone in air where it has a very spe cific sharp odor somewhat resembling chlorine bleach. Exposure of 0.1 to 1 mol/mo l produces headaches, burning eyes, and irritation to the respiratory passages.[ 6] Even low concentrations of ozone in air are very destructive to organic mater ials such as latex, plastics, and animal lung tissue. Ozone is diamagnetic, which means that its electrons are all paired. In contrast , O2 is paramagnetic, containing two unpaired electrons. [edit] StructureAccording to experimental evidence from microwave spectroscopy, ozone is a bent molecule, with C2v symmetry (similar to the water molecule). The O O distances are 127.2 pm. The O O O angle is 116.78.[7] The central atom is sp hybridized with one lone pair. Ozone is a polar molecule with a dipole moment of 0.53 D.[8] The bonding can be expressed as a resonance hybrid with a single bon d on one side and double bond on the other producing an overall bond order of 1. 5 for each side. [edit] ReactionsOzone is a powerful oxidizing agent, far stronger than O2. It is also unstable at high concentrations, decaying to ordinary diatomic oxygen (wit h a half life of about half an hour in atmospheric conditions):[9] 2 O3 3 O2 This reaction proceeds more rapidly with increasing temperature and increased pr
[ d t] Phys cal prop rt d much mor solubl fluorocarbo s, wh r t orm a dark blue liquid. ing point, because both . At temperatures below
sO o s a pal blu gas, sl ghtly solubl wat r a rt o -polar solv ts such as carbo t trachlor d or forms a blu solut o . At 161 K (112 C), it condenses to f It is dangerous to allow this liquid to warm to its boil concentrated gaseous ozone and liquid ozone can detonate 80 K (193 C), it forms a violet black solid.[5]
6 H alth ff cts 6.1 A r pollut o 6.2 Phys ology 6.3 Saf ty r gulat o s 7 Product o 7.1 Coro a d scharg m 7.2 Ultrav ol t l ght 7.3 Cold plasma 7.4 El ctrolyt c 7.5 Sp c al co s d rat 7.6 I c d tal product 7.7 Laboratory product 8 Appl cat o s 8.1 I dustry 8.2 Co sum rs 8.3 Aquacultur 8.4 Agr cultur 9 S also 10 R f r c s 11 Furth r r ad g 12 Ext r al l ks
thod
o s o o
essure. Deflagration of ozone can be triggered by a spark, and can occur in ozon e concentrations of 10 wt% or higher.[10] [edit] With metalsOzone will oxidize most metals (except gold, platinum, and iri dium) to oxides of the metals in their highest oxidation state. For example: 2 Cu+ + 2 H3O+ + O3 2 Cu2+ + 3 H2O + O2 [edit] With nitrogen and carbon compoundsOzone also oxidizes nitric oxide to nit rogen dioxide: NO + O3 NO2 + O2 This reaction is accompanied by chemiluminescence. The NO2 can be further oxidiz ed: NO2 + O3 NO3 + O2 The NO3 formed can react with NO2 to form N2O5: Solid nitryl perchlorate can be made from NO2, ClO2, and O3 gases: 2 NO2 + 2 ClO2 + 2 O3 2 NO2ClO4 + O2 Ozone does not react with ammonium salts, but it oxidizes ammonia to ammonium ni trate: 2 NH3 + 4 O3 NH4NO3 + 4 O2 + H2O Ozone reacts with carbon to form carbon dioxide, even at room temperature: C + 2 O3 CO2 + 2 O2 [edit] With sulfur compoundsOzone oxidizes sulfides to sulfates. For example, le ad(II) sulfide is oxidised to lead(II) sulfate: PbS + 4 O3 PbSO4 + 4 O2 Sulfuric acid can be produced from ozone, water and either elemental sulfur or s ulfur dioxide: S + H2O + O3 H2SO4 3 SO2 + 3 H2O + O3 3 H2SO4 In the gas phase, ozone reacts with hydrogen sulfide to form sulfur dioxide: H2S + O3 SO2 + H2O In an aqueous solution, however, two competing simultaneous reactions occur, one to produce elemental sulfur, and one to produce sulfuric acid: H2S + O3 S + O2 + H2O 3 H2S + 4 O3 3 H2SO4 [edit] With alkenes and alkynesMain article: ozonolysis Alkenes can be oxidatively cleaved by ozone, in a process called ozonolysis, giv ing alcohols, aldehydes, ketones, and carboxylic acids, depending on the second step of the workup.
Usually ozonolysis is carried out in a solution of dichloromethane, at a tempera ture of 78oC. After a sequence of cleavage and rearrangement, an organic ozonid e is formed. With reductive workup (e.g. Zinc in acetic acid or dimethyl sulfide ), ketones and aldehydes will be formed, with oxidative workup (e.g. aqueous or alcoholic hydrogen peroxide), carboxylic acids will be formed.[11] [edit] Other substratesAll three atoms of ozone may also react, as in the reacti on of tin(II) chloride with hydrochloric acid and ozone:
3 SnCl2 + 6 HCl + O3 3 SnCl4 + 3 H2O Iodine perchlorate can be made by treating iodine dissolved in cold anhydrous pe rchloric acid with ozone: I2 + 6 HClO4 + O3 2 I(ClO4)3 + 3 H2O [edit] CombustionOzone can be used for combustion reactions and combusting gases ; ozone provides higher temperatures than combusting in dioxygen (O2). The follo wing is a reaction for the combustion of carbon subnitride which can also cause higher temperatures: 3 C4N2 + 4 O3 12 CO + 3 N2 Ozone can react at cryogenic temperatures. At 77 K (196 C), atomic hydrogen reacts with liquid ozone to form a hydrogen superoxide radical, which dimerizes:[12] H + O3 HO2 + O 2 HO2 H2O4 [edit] Reduction to ozonidesReduction of ozone gives the ozonide anion, O3 . Deri vatives of this anion are explosive and must be stored at cryogenic temperatures . Ozonides for all the alkali metals are known. KO3, RbO3, and CsO3 can be prepa red from their respective superoxides: KO2 + O3 KO3 + O2 Although KO3 can be formed as above, it can also be formed from potassium hydrox ide and ozone:[13] 2 KOH + 5 O3 2 KO3 + 5 O2 + H2O NaO3 and LiO3 must be prepared by action of CsO3 in liquid NH3 on an ion exchang e resin containing Na+ or Li+ ions:[14] CsO3 + Na+ Cs+ + NaO3 A solution of calcium in ammonia reacts with ozone to give to ammonium ozonide a nd not calcium ozonide:[12] 3 Ca + 10 NH3 + 6 O3 Ca6NH3 + Ca(OH)2 + Ca(NO3)2 + 2 NH4O3 + 2 O2 + H2 [edit] ApplicationsOzone can be used to remove manganese from water, forming a p recipitate which can be filtered: 2 Mn2+ + 2 O3 + 4 H2O 2 MnO(OH)2 (s) + 2 O2 + 4 H+ Ozone will also detoxify cyanides by converting them to cyanates, which are a th ousand times less toxic.[citation needed] CN + O3 CNO + O2 Ozone will also completely decompose urea:[15] (NH2)2CO + O3 N2 + CO2 + 2 H2O [edit] Ozone in Earth's atmosphere The distribution of atmospheric ozone in partial pressure as a function of altit ude Concentration of ozone as measured by the Nimbus 7 satellite Total ozone concentration in June 2000 as measured by EP TOMS satellite instrume ntThe standard way to express total ozone levels (the amount of ozone in a verti cal column) in the atmosphere is by using Dobson units. Point measurements are r eported as mole fractions in nmol/mol (parts per billion, ppb) or as concentrati ons in g/m3. [edit] Ozone layerMain article: Ozone layer [edit] Location and productionThe highest levels of ozone in the atmosphere are in the stratosphere, in a region also known as the ozone layer between about 10 km and 50 km above the surface (or between about 6 and 31 miles). However, even in this "layer" the ozone concentrations are only two to eight parts per million
, so most of the oxygen there remains of the dioxygen type. Ozone in the stratosphere is mostly produced from short wave ultraviolet rays (i n the UVC band) reacting with oxygen: O2 + photon (radiation < 240 nm) 2 O O + O2 + M O3 + M where "M" denotes the third body that carries off the excess energy of the react ion. The thus produced ozone is destroyed by the reaction with atomic oxygen: O3 + O 2 O2 The latter reaction is catalysed by the presence of certain free radicals, of wh ich the most important are hydroxyl (OH), nitric oxide (NO) and atomic chlorine (Cl) and bromine (Br). In recent decades the amount of ozone in the stratosphere has been declining mostly because of emissions of CFCs and similar chlorinated and brominated organic molecules, which have increased the concentration of ozon e depleting catalysts above the natural background. See also: Ozone oxygen cycle and Ozone depletion [edit] Importance to surface dwelling life on Earth Levels of ozone at various altitudes and blocking of different bands of ultravio let radiation. Essentially all UVC (100 280 nm) is blocked by dioxygen (from 100 200 nm) or by ozone (200 280 nm) in the atmosphere. The shorter portion of this band and even more energetic UV causes the formation of the ozone layer, when s ingle oxygen atoms produced by UV photolysis of dioxygen (below 240 nm) react wi th more dioxygen. The ozone layer itself then blocks most, but not quite all, su nburn producing UVB (280 315 nm). The band of UV closest to visible light, UVA ( 315 400 nm), is hardly affected by ozone, and most of it reaches the ground.Ozon e in the ozone layer filters out sunlight wavelengths from about 200 nm UV rays to 315 nm, with ozone peak absorption at about 250 nm.[16] This ozone UV absorpt ion is important to life, since it extends the absorption of UV by ordinary oxyg en and nitrogen in air (which absorbs all wavelengths < 200 nm) through the lowe r UV C (200 nm 280 nm) and the entire UV B band (280 nm 315 nm). The small unabs orbed part that remains of UV B after passage through ozone causes sunburn in hu mans, and direct DNA damage in living tissues in both plants and animals. Ozone' s effect on mid range UV B rays is illustrated by its effect on UV B at 290 nm, which has a radiation intensity 350 million times as powerful at the top of the atmosphere as at the surface. Nevertheless, enough of UV B radiation at similar frequency reaches the ground to cause some sunburn, and these same wavelengths a re also among those responsible for the production of vitamin D in humans. The ozone layer has little effect on the longer UV wavelengths called UV A (315 nm 400 nm), but this radiation does not cause sunburn or direct DNA damage, an d while it probably does cause long term skin damage in certain humans, it is no t as dangerous to plants and to the health of surface dwelling organisms on Eart h in general (see ultraviolet for more information on near ultraviolet). [edit] Low level ozoneMain articles: Tropospheric ozone and Photochemical smog Low level ozone (or tropospheric ozone) is an atmospheric pollutant.[17] It is n ot emitted directly by car engines or by industrial operations, but formed by th e reaction of sunlight on air containing hydrocarbons and nitrogen oxides that r eact to form ozone directly at the source of the pollution or many kilometers do wn wind. Ozone reacts directly with some hydrocarbons such as aldehydes and thus begins t heir removal from the air, but the products are themselves key components of smo g. Ozone photolysis by UV light leads to production of the hydroxyl radical OH a nd this plays a part in the removal of hydrocarbons from the air, but is also th e first step in the creation of components of smog such as peroxyacyl nitrates w hich can be powerful eye irritants. The atmospheric lifetime of tropospheric ozo
ne is about 22 days; its main removal mechanisms are being deposited to the grou nd, the above mentioned reaction giving OH, and by reactions with OH and the per oxy radical HO2.[18] There is evidence of significant reduction in agricultural yields because of inc reased ground level ozone and pollution which interferes with photosynthesis and stunts overall growth of some plant species.[19][20] The United States Environm ental Protection Agency is proposing a secondary regulation to reduce crop damag e, in addition to the primary regulation designed for the protection of human he alth. Certain examples of cities with elevated ozone readings are Houston, Texas, and Mexico City, Mexico. Houston has a reading of around 41 nmol/mol, while Mexico C ity is far more hazardous, with a reading of about 125 nmol/mol.[20] [edit] Ozone cracking Ozone cracking in natural rubber tubingOzone gas attacks any polymer possessing olefinic or double bonds within its chain structure, such as natural rubber, nit rile rubber, and styrene butadiene rubber. Products made using these polymers ar e especially susceptible to attack, which causes cracks to grow longer and deepe r with time, the rate of crack growth depending on the load carried by the produ ct and the concentration of ozone in the atmosphere. Such materials can be prote cted by adding antiozonants, such as waxes, which bond to the surface to create a protective film or blend with the material and provide long term protection. O zone cracking used to be a serious problem in car tires for example, but the pro blem is now seen only in very old tires. On the other hand, many critical produc ts like gaskets and O rings may be attacked by ozone produced within compressed air systems. Fuel lines are often made from reinforced rubber tubing and may als o be susceptible to attack, especially within engine compartments where low leve ls of ozone are produced from electrical equipment. Storing rubber products in c lose proximity to DC electric motors can accelerate the rate at which ozone crac king occurs. The commutator of the motor creates sparks which in turn produce oz one. [edit] Ozone as a greenhouse gasAlthough ozone was present at ground level befor e the Industrial Revolution, peak concentrations are now far higher than the pre industrial levels, and even background concentrations well away from sources of pollution are substantially higher.[21][22] This increase in ozone is of furthe r concern because ozone present in the upper troposphere acts as a greenhouse ga s, absorbing some of the infrared energy emitted by the earth. Quantifying the g reenhouse gas potency of ozone is difficult because it is not present in uniform concentrations across the globe. However, the most widely accepted scientific a ssessments relating to climate change (e.g. the Intergovernmental Panel on Clima te Change Third Assessment Report[23]) suggest that the radiative forcing of tro pospheric ozone is about 25% that of carbon dioxide. The annual global warming potential of tropospheric ozone is between 9181022 tons carbon dioxide equivalent/tons tropospheric ozone. This means on a per molecule basis, ozone in the troposphere has a radiative forcing effect roughly 1,000 ti mes as strong as carbon dioxide. However, tropospheric ozone is a short lived gr eenhouse gas, which decays in the atmosphere much more quickly than carbon dioxi de. This means that over a 20 year horizon, the global warming potential of trop ospheric ozone is much less, roughly 62 to 69 tons carbon dioxide equivalent / t ons tropospheric ozone.[24] Because of its short lived nature, tropospheric ozone does not have strong globa l effects, but has very strong radiative forcing effects on regional scales. In fact, there are regions of the world where tropospheric ozone has a radiative fo rcing up to 150% of carbon dioxide.[25]
[edit] Health effectsSee also: Environmental impact of the coal industry [edit] Air pollution Red Alder leaf, showing the typical discolouration caused by ozone pollution[26] Signboard in Gulfton, Houston indicating an ozone watchGround level ozone is cre ated near the Earth's surface by the action of daylight UV rays on a group of po llutants called ozone precursors. There is a great deal of evidence to show that ground level ozone can harm lung function and irritate the respiratory system.[ 17][27] Exposure to ozone and the pollutants that produce it is linked to premat ure death, asthma, bronchitis, heart attack, and other cardiopulmonary problems. [citation needed] Long term exposure to ozone has been shown to increase risk of death from respir atory illness. A study of 450,000 people living in United States cities showed a significant correlation between ozone levels and respiratory illness over the 1 8 year follow up period. The study revealed that people living in cities with hi gh ozone levels such as Houston or Los Angeles had an over 30% increased risk of dying from lung disease.[28][29] Air quality guidelines such as those from the World Health Organization, the Uni ted States Environmental Protection Agency (EPA) and the European Union are base d on detailed studies designed to identify the levels that can cause measurable ill health effects. According to scientists with the EPA, susceptible people can be adversely affect ed by ozone levels as low as 40 nmol/mol.[30] In the EU, the current target value for ozone concentrations is 120 g/m which is a bout 60 nmol/mol. This target applies to all member states in accordance with Di rective 2008/50/EC.[31] Ozone concentration is measured as a maximum daily mean of 8 hour averages and the target should not be exceeded on more than 25 calenda r days per year, starting from January 2010. Whilst the directive requires in th e future a strict compliance with 120 g/m limit (i.e. mean ozone concentration not to be exceeded on any day of the year), there is no date set for this requireme nt and this is treated as a long term objective. [32] The Clean Air Act directs the EPA to set National Ambient Air Quality Standards for several pollutants, including ground level ozone, and counties out of compli ance with these standards are required to take steps to reduce their levels. In May 2008, the EPA lowered its ozone standard from 80 nmol/mol to 75 nmol/mol. Th is proved controversial, since the Agency's own scientists and advisory board ha d recommended lowering the standard to 60 nmol/mol, and the World Health Organiz ation recommends 51 nmol/mol. Many public health and environmental groups also s upported the 60 nmol/mol standard.[33] On January 7, 2010, the U.S. Environmenta l Protection Agency (EPA) announced proposed revisions to the National Ambient A ir Quality Standard (NAAQS) for the pollutant ozone, the principal component of smog: ... EPA proposes that the level of the 8 hour primary standard, which was set at 0.075 mol/mol in the 2008 final rule, should instead be set at a lower level wit hin the range of 0.060 to 0.070 mol/mol, to provide increased protection for chil dren and other at risk populations against an array of O3- related adverse health ef fects that range from decreased lung function and increased respiratory symptoms to serious indicators of respiratory morbidity including emergency department v isits and hospital admissions for respiratory causes, and possibly cardiovascula r-related morbidity as well as total non- accidental and cardiopulmonary mortali ty....[34] The EPA has developed an Air Quality Index (AQI) to help explain air pollution l evels to the general public. Under the current standards, eight-hour average ozo ne mole fractions of 85 to 104 nmol/mol are described as "unhealthy for sensitiv
e groups," 105 nmol/mol to 124 nmol/mol as "unhealthy," and 125 nmol/mol to 404 nmol/mol as "very unhealthy."[35] Ozone can also be present in indoor air pollution, partly as a result of electro nic equipment such as photocopiers. A connection has also been known to exist be tween the increased pollen, fungal spores, and ozone caused by thunderstorms and hospital admissions of asthma sufferers.[36] In the Victorian era, one British folk myth held that the smell of the sea was c aused by ozone. In fact, the characteristic "smell of the sea" is caused by dime thyl sulfide a chemical generated by phytoplankton. Victorian British folk consi dered the resulting smell "bracing," but in high concentrations, dimethyl sulfid e is actually toxic.[37] [edit] PhysiologySee also: trioxidane Ozone, along with reactive forms of oxygen such as superoxide, singlet oxygen, h ydrogen peroxide, and hypochlorite ions, is naturally produced by white blood ce lls and other biological systems (such as the roots of marigolds) as a means of destroying foreign bodies. Ozone reacts directly with organic double bonds. Also , when ozone breaks down to dioxygen it gives rise to oxygen free radicals, whic h are highly reactive and capable of damaging many organic molecules. Moreover, it is believed that the powerful oxidizing properties of ozone may be a contribu ting factor of inflammation. The cause-and-effect relationship of how the ozone is created in the body and what it does is still under consideration and still s ubject to various interpretations, since other body chemical processes can trigg er some of the same reactions. A team headed by Dr. Paul Wentworth Jr. of the De partment of Chemistry at the Scripps Research Institute has shown evidence linki ng the antibody-catalyzed water-oxidation pathway of the human immune response t o the production of ozone. In this system, ozone is produced by antibody-catalyz ed production of trioxidane from water and neutrophil-produced singlet oxygen.[3 8] When inhaled, ozone reacts with compounds lining the lungs to form specific, cho lesterol-derived metabolites that are thought to facilitate the build-up and pat hogenesis of atherosclerotic plaques (a form of heart disease). These metabolite s have been confirmed as naturally occurring in human atherosclerotic arteries a nd are categorized into a class of secosterols termed atheronals, generated by o zonolysis of cholesterol s double bond to form a 5,6 secosterol[39] as well as a secondary condensation product via aldolization.[40] Ozone has been implicated to have an adverse effect on plant growth: "... ozone reduced total chlorophylls, carotenoid and carbohydrate concentration, and incre ased 1-aminocyclopropane-1-carboxylic acid (ACC) content and ethylene production . In treated plants, the ascorbate leaf pool was decreased, while lipid peroxida tion and solute leakage were significantly higher than in ozone-free controls. T he data indicated that ozone triggered protective mechanisms against oxidative s tress in citrus."[41] [edit] Safety regulationsDue to the strongly oxidizing properties of ozone, ozon e is a primary irritant, affecting especially the eyes and respiratory systems a nd can be hazardous at even low concentrations. The Canadian Center for Occupati on Safety and Health reports that: "Even very low concentrations of ozone can be harmful to the upper respiratory t ract and the lungs. The severity of injury depends on both by the concentration of ozone and the duration of exposure. Severe and permanent lung injury or death could result from even a very short-term exposure to relatively low concentrati ons." [42] To protect workers potentially exposed to ozone, U.S. Occupational Safety and He
alth Administration has established a permissible exposure limit (PEL) of 0.1 mol /mol (29 CFR 1910.1000 table Z 1), calculated as an 8 hour time weighted average . Higher concentrations are especially hazardous and NIOSH has established an Im mediately Dangerous to Life and Health Limit (IDLH) of 5 mol/mol.[43] Work enviro nments where ozone is used or where it is likely to be produced should have adeq uate ventilation and it is prudent to have a monitor for ozone that will alarm i f the concentration exceeds the OSHA PEL. Continuous monitors for ozone are avai lable from several suppliers. Elevated ozone exposure can occur on passenger aircraft, with levels depending o n altitude and atmospheric turbulence.[44] United States Federal Aviation Author ity regulations set a limit of 250 nmol/mol with a maximum four hour average of 100 nmol/mol.[45] Some planes are equipped with ozone converters in the ventilat ion system to reduce passenger exposure.[44] [edit] ProductionOzone often forms in nature under conditions where O2 will not react.[6] Ozone used in industry is measured in mol/mol (ppm, parts per million), nmol/mol (ppb, parts per billion), g/m3, mg/hr (milligrams per hour) or weight p ercent. The regime of applied concentrations ranges from 1 to 5% in air and from 6 to 14% in oxygen for older generation methods. New electrolytic methods can a chieve up 20 to 30% dissolved ozone concentrations in output water. Temperature and humidity plays a large role in how much ozone is being produced using traditional generation methods such as corona discharge and ultraviolet li ght. Old generation methods will produce less than 50% its nominal capacity if o perated with humid ambient air than when it operates in very dry air. New genera tors using electrolytic methods can achieve higher purity and dissolution throug h using water molecules as the source of ozone production. [edit] Corona discharge methodThis is the most common type of ozone generator fo r most industrial and personal uses. While variations of the "hot spark" coronal discharge method of ozone production exist, including medical grade and industr ial grade ozone generators, these units usually work by means of a corona discha rge tube.[46] They are typically cost effective and do not require an oxygen sou rce other than the ambient air to produce ozone concentrations of 36%. Fluctuatio ns in ambient air, due to weather or other environmental conditions, cause varia bility in ozone production. However, they also produce nitrogen oxides as a by p roduct. Use of an air dryer can reduce or eliminate nitric acid formation by rem oving water vapor and increase ozone production. Use of an oxygen concentrator c an further increase the ozone production and further reduce the risk of nitric a cid formation by removing not only the water vapor, but also the bulk of the nit rogen. [edit] Ultraviolet lightUV ozone generators, or vacuum ultraviolet (VUV) ozone g enerators, employ a light source that generates a narrow band ultraviolet light, a subset of that produced by the Sun. The Sun's UV sustains the ozone layer in the stratosphere of Earth.[47] While standard UV ozone generators tend to be less expensive,[clarification need ed] they usually produce ozone with a concentration of about 0.5% or lower. Anot her disadvantage of this method is that it requires the air (oxygen) to be expos ed to the UV source for a longer amount of time, and any gas that is not exposed to the UV source will not be treated. This makes UV generators impractical for use in situations that deal with rapidly moving air or water streams (in duct ai r sterilization, for example). Production of ozone is one of the potential dange rs of ultraviolet germicidal irradiation. VUV ozone generators are used in swimm ing pool and spa applications ranging to millions of gallons of water. VUV ozone generators, unlike corona discharge generators, do not produce harmful nitrogen by products and also unlike corona discharge systems, VUV ozone generators work extremely well in humid air environments. There is also not normally a need for
expensive off gas mechanisms, and no need for air driers or oxygen concentrator s which require extra costs and maintenance. [edit] Cold plasmaIn the cold plasma method, pure oxygen gas is exposed to a pla sma created by dielectric barrier discharge. The diatomic oxygen is split into s ingle atoms, which then recombine in triplets to form ozone. Cold plasma machines utilize pure oxygen as the input source and produce a maxim um concentration of about 5% ozone. They produce far greater quantities of ozone in a given space of time compared to ultraviolet production. However, because c old plasma ozone generators are very expensive, they are found less frequently t han the previous two types. The discharges manifest as filamentary transfer of electrons (micro discharges) in a gap between two electrodes. In order to evenly distribute the micro dischar ges, a dielectric insulator must be used to separate the metallic electrodes and to prevent arcing. Some cold plasma units also have the capability of producing short lived allotro pes of oxygen which include O4, O5, O6, O7, etc. These species are even more rea ctive than ordinary O3.[citation needed] [edit] ElectrolyticElectrolytic ozone generation (EOG) splits water molecules in to H2, O2, and O3. In most EOG methods, the hydrogen gas will be removed to leav e oxygen and ozone as the only reaction products. Therefore, EOG can achieve hig her dissolution in water without other competing gases found in corona discharge method, such as nitrogen gases present in ambient air. This method of generatio n can achieve concentrations of 2030% and is independent of air quality because w ater is used as the starting substrate. [edit] Special considerationsOzone cannot be stored and transported like other i ndustrial gases (because it quickly decays into diatomic oxygen) and must theref ore be produced on site. Available ozone generators vary in the arrangement and design of the high voltage electrodes. At production capacities higher than 20 k g per hour, a gas/water tube heat exchanger may be utilized as ground electrode and assembled with tubular high voltage electrodes on the gas side. The regime o f typical gas pressures is around 2 bar absolute in oxygen and 3 bar absolute in air. Several megawatts of electrical power may be installed in large facilities , applied as one phase AC current at 50 to 8000 Hz and peak voltages between 3,0 00 and 20,000 volts. Applied voltage is usually inversely related to the applied frequency. The dominating parameter influencing ozone generation efficiency is the gas temp erature, which is controlled by cooling water temperature and/or gas velocity. T he cooler the water, the better the ozone synthesis. The lower the gas velocity, the higher the concentration (but the lower the net ozone produced). At typical industrial conditions, almost 90% of the effective power is dissipated as heat and needs to be removed by a sufficient cooling water flow. Because of the high reactivity of ozone, only a few materials may be used like s tainless steel (quality 316L), titanium, aluminium (as long as no moisture is pr esent), glass, polytetrafluorethylene, or polyvinylidene fluoride. Viton may be used with the restriction of constant mechanical forces and absence of humidity (humidity limitations apply depending on the formulation). Hypalon may be used w ith the restriction that no water come in contact with it, except for normal atm ospheric levels. Embrittlement or shrinkage is the common mode of failure of ela stomers with exposure to ozone. Ozone cracking is the common mode of failure of elastomer seals like O rings. Silicone rubbers are usually adequate for use as gaskets in ozone concentrations
below 1 wt%, such as in equipment for accelerated aging of rubber samples. [edit] Incidental productionOzone may be formed from O2 by electrical discharges and by action of high energy electromagnetic radiation. Unsuppressed arcing bre aks down the chemical bonds of the atmospheric oxygen surrounding the contacts [ O2 2O]. Free ions of oxygen in and around the arc recombine to create ozone [O3] .[48] Certain electrical equipment generate significant levels of ozone. This is especially true of devices using high voltages, such as ionic air purifiers, la ser printers, photocopiers, tasers and arc welders. Electric motors using brushe s can generate ozone from repeated sparking inside the unit. Large motors that u se brushes, such as those used by elevators or hydraulic pumps, will generate mo re ozone than smaller motors. Ozone is similarly formed in the Catatumbo lightni ng storms phenomenon on the Catatumbo River in Venezuela, which helps to repleni sh ozone in the upper troposphere. It is the world's largest single natural gene rator of ozone, lending calls for it to be designated a UNESCO World Heritage Si te.[49] [edit] Laboratory productionIn the laboratory, ozone can be produced by electrol ysis using a 9 volt battery, a pencil graphite rod cathode, a platinum wire anod e and a 3 molar sulfuric acid electrolyte.[50] The half cell reactions taking pl ace are: 3 H2O O3 + 6 H+ + 6 e (Eo = 1.53 V) 6 H+ + 6 e 3 H2 (Eo = 0 V) 2 H2O O2 + 4 H+ + 4 e (Eo = 1.23 V) In the net reaction, three equivalents of water are converted into one equivalen t of ozone and three equivalents of hydrogen. Oxygen formation is a competing re action. It can also be "prepared" by high voltage arc. This can be done with an apparatu s consisting of two concentric glass tubes sealed together at the top, with in a nd out spigots at the top and bottom of the outer tube. The inner core should ha ve a length of metal foil inserted into it connected to one side of the power so urce. The other side of the power source should be connected to another piece of foil wrapped around the outer tube. Dry O2 should be run through the tube in on e spigot. As the O2 is run through one spigot into the apparatus and high voltag e is applied to the foil leads, electricity will discharge between the dry dioxy gen in the middle and form O3 and O2 out the other spigot. The reaction can be s ummarized as follows:[6] 3 O2 electricity 2 O3 [edit] Applications[edit] IndustryThe largest use of ozone is in the preparation of pharmaceuticals, synthetic lubricants, and many other commercially useful or ganic compounds, where it is used to sever carbon carbon bonds.[6] It can also b e used for bleaching substances and for killing microorganisms in air and water sources.[51] Many municipal drinking water systems kill bacteria with ozone inst ead of the more common chlorine.[52] Ozone has a very high oxidation potential.[ 53] Ozone does not form organochlorine compounds, nor does it remain in the wate r after treatment. Ozone can form the suspected carcinogen [bromate] in source w ater with high bromide concentrations. The Safe Drinking Water Act mandates that these systems introduce an amount of chlorine to maintain a minimum of 0.2 mol/m ol residual free chlorine in the pipes, based on results of regular testing. Whe re electrical power is abundant, ozone is a cost effective method of treating wa ter, since it is produced on demand and does not require transportation and stor age of hazardous chemicals. Once it has decayed, it leaves no taste or odor in d rinking water. Although low levels of ozone have been advertised to be of some disinfectant use in residential homes, the concentration of ozone in dry air required to have a rapid, substantial effect on airborne pathogens exceeds safe levels recommended
by the U.S. Occupational Safety and Health Administration and Environmental Prot ection Agency. Humidity control can vastly improve both the killing power of the ozone and the rate at which it decays back to oxygen (more humidity allows more effectiveness). Spore forms of most pathogens are very tolerant of atmospheric ozone in concentrations where asthma patients start to have issues. Industrially, ozone is used to: Disinfect laundry in hospitals, food factories, care homes etc.;[54] Disinfect water in place of chlorine[6] Deodorize air and objects, such as after a fire. This process is extensively use d in fabric restoration Kill bacteria on food or on contact surfaces;[55] Sanitize swimming pools and spas Kill insects in stored grain[56] Scrub yeast and mold spores from the air in food processing plants; Wash fresh fruits and vegetables to kill yeast, mold and bacteria;[55] Chemically attack contaminants in water (iron, arsenic, hydrogen sulfide, nitrit es, and complex organics lumped together as "colour"); Provide an aid to flocculation (agglomeration of molecules, which aids in filtra tion, where the iron and arsenic are removed); Manufacture chemical compounds via chemical synthesis[57] Clean and bleach fabrics (the former use is utilized in fabric restoration; the latter use is patented);[citation needed] Assist in processing plastics to allow adhesion of inks; Age rubber samples to determine the useful life of a batch of rubber; Eradicate water borne parasites such as Giardia lamblia and Cryptosporidium in s urface water treatment plants. Ozone is a reagent in many organic reactions in the laboratory and in industry. Ozonolysis is the cleavage of an alkene to carbonyl compounds. Many hospitals around the world use large ozone generators to decontaminate oper ating rooms between surgeries. The rooms are cleaned and then sealed airtight be fore being filled with ozone which effectively kills or neutralizes all remainin g bacteria.[58] Ozone is used as an alternative to chlorine or chlorine dioxide in the bleaching of wood pulp.[59] It is often used in conjunction with oxygen and hydrogen pero xide to eliminate the need for chlorine containing compounds in the manufacture of high quality, white paper.[60] Ozone can be used to detoxify cyanide wastes (for example from gold and silver m ining) by oxidizing cyanide to cyanate and eventually to carbon dioxide.[61] [edit] ConsumersDevices generating high levels of ozone, some of which use ioniz ation, are used to sanitize and deodorize uninhabited buildings, rooms, ductwork , woodsheds, and boats and other vehicles. In the U.S., air purifiers emitting low levels of ozone have been sold. This kin d of air purifier is sometimes claimed to imitate nature's way of purifying the air without filters and to sanitize both it and household surfaces. The United S tates Environmental Protection Agency (EPA) has declared that there is "evidence to show that at concentrations that do not exceed public health standards, ozon e is not effective at removing many odor causing chemicals" or "viruses, bacteri a, mold, or other biological pollutants." Furthermore, its report states that "r esults of some controlled studies show that concentrations of ozone considerably higher than these [human safety] standards are possible even when a user follow s the manufacturers operating instructions."[62] The government successfully sued one company in 1995, ordering it to stop repeating health claims without suppor ting scientific studies.
Ozonated water is used to launder clothes and to sanitize food, drinking water, and surfaces in the home. According to the U.S. Food and Drug Administration (FD A), it is "amending the food additive regulations to provide for the safe use of ozone in gaseous and aqueous phases as an antimicrobial agent on food, includin g meat and poultry." Studies at California Polytechnic University demonstrated t hat 0.3 mol/mol levels of ozone dissolved in filtered tapwater can produce a redu ction of more than 99.99% in such food borne microorganisms as salmonella, E. co li 0157:H7, and Campylobacter. This quantity is 20,000 times the WHO recommended limits stated above.[55][63] Ozone can be used to remove pesticide residues fro m fruits and vegetables.[64][65] Ozone is used in homes and hot tubs to kill bacteria in the water and to reduce the amount of chlorine or bromine required by reactivating them to their free st ate. Since ozone does not remain in the water long enough, ozone by itself is in effective at preventing cross contamination among bathers and must be used in co njunction with halogens. Gaseous ozone created by ultraviolet light or by corona discharge is injected into the water.[66] Ozone is also widely used in treatment of water in aquariums and fish ponds. Its use can minimize bacterial growth, control parasites, eliminate transmission of some diseases, and reduce or eliminate "yellowing" of the water. Ozone must not come in contact with fish's gill structures. Natural salt water (with life form s) provides enough "instantaneous demand" that controlled amounts of ozone activ ate bromide ion to hypobromous acid, and the ozone entirely decays in a few seco nds to minutes. If oxygen fed ozone is used, the water will be higher in dissolv ed oxygen, fish's gill structures will atrophy and they will become dependent on higher dissolved oxygen levels. [edit] AquacultureOzone can be used in aquaculture to facilitate organic breakdo wn. It is added to recirculating systems to reduce nitrite levels[67] through co nversion into nitrate. If nitrite levels in the water are high, nitrites will al so accumulate in the blood and tissues of fish, where it interferes with oxygen transport (it causes oxidation of the heme group of haemoglobin from ferrous(Fe2 +) to ferric (Fe3+), making haemoglobin unable to bind O2[68]). Despite these ap parent positive effects, ozone use in recirculation systems has been linked to r educing the level of bioavailable iodine in salt water systems, resulting in iod ine deficiency symptoms such as goitre and decreased growth in Senegalese sole ( Solea senegalensis) larvae.[69] Ozonate seawater is used for surface disinfection of haddock and Atlantic halibu t eggs against nodavirus. Nodavirus is a lethal and vertically transmitted virus which causes severe mortality in fish. Haddock eggs should not be treated with high ozone level as eggs so treated did not hatch and died after 34 days.[70] [edit] AgricultureOzone application on freshly cut pineapple and banana shows in crease in flavonoids and total phenol contents when exposure is up to 20 minutes . Decrease in ascorbic acid content is observed but the positive effect on total phenol content and flavonoids can overcome the negative effect.[71] Tomatoes up on treatment with ozone shows an increase in -carotene, lutein and lycopene.[72] However, ozone application on straw erries in pre-harvest period shows decrease in ascor ic acid content.[73] Ozone facilitates the extraction of some heavy metals from soil using EDTA. EDTA forms strong, water-solu le coordination compounds with some heavy metals (P , Zn) there y making it possi le to dissolve them out from contaminated soil. If c ontaminated soil is pre-treated with ozone, the extraction efficacy of P , Am an d Pu increases y 1128.9%,[74] 43.5%[75] and 50.7%[75] respectivel
También podría gustarte
- Interpretation of StatutesDocumento55 páginasInterpretation of Statutesapi-382308896% (70)
- Costitution of IndiaDocumento341 páginasCostitution of IndiaJITENDER PAL SINGHAún no hay calificaciones
- JurisprudenceDocumento12 páginasJurisprudenceJITENDER PAL SINGHAún no hay calificaciones
- 4 Law of PartitionDocumento52 páginas4 Law of PartitionJITENDER PAL SINGH60% (5)
- Moot Court TipsDocumento7 páginasMoot Court TipsJITENDER PAL SINGHAún no hay calificaciones
- Moot Court TipsDocumento7 páginasMoot Court TipsJITENDER PAL SINGHAún no hay calificaciones
- Pubint Williams f01 2Documento52 páginasPubint Williams f01 2Luke Concepcion-ButayAún no hay calificaciones
- Features of Water (Prevention and Control) Act 1974Documento5 páginasFeatures of Water (Prevention and Control) Act 1974JITENDER PAL SINGH100% (1)
- Jurisprudence Notes Lecture 1Documento5 páginasJurisprudence Notes Lecture 1Usama AlviAún no hay calificaciones
- The Yellow House: A Memoir (2019 National Book Award Winner)De EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Calificación: 4 de 5 estrellas4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeCalificación: 4 de 5 estrellas4/5 (5795)
- Shoe Dog: A Memoir by the Creator of NikeDe EverandShoe Dog: A Memoir by the Creator of NikeCalificación: 4.5 de 5 estrellas4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureCalificación: 4.5 de 5 estrellas4.5/5 (474)
- Grit: The Power of Passion and PerseveranceDe EverandGrit: The Power of Passion and PerseveranceCalificación: 4 de 5 estrellas4/5 (588)
- On Fire: The (Burning) Case for a Green New DealDe EverandOn Fire: The (Burning) Case for a Green New DealCalificación: 4 de 5 estrellas4/5 (74)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryCalificación: 3.5 de 5 estrellas3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceCalificación: 4 de 5 estrellas4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe EverandNever Split the Difference: Negotiating As If Your Life Depended On ItCalificación: 4.5 de 5 estrellas4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe EverandThe Little Book of Hygge: Danish Secrets to Happy LivingCalificación: 3.5 de 5 estrellas3.5/5 (400)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersCalificación: 4.5 de 5 estrellas4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaDe EverandThe Unwinding: An Inner History of the New AmericaCalificación: 4 de 5 estrellas4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnDe EverandTeam of Rivals: The Political Genius of Abraham LincolnCalificación: 4.5 de 5 estrellas4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyCalificación: 3.5 de 5 estrellas3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaCalificación: 4.5 de 5 estrellas4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerDe EverandThe Emperor of All Maladies: A Biography of CancerCalificación: 4.5 de 5 estrellas4.5/5 (271)
- Rise of ISIS: A Threat We Can't IgnoreDe EverandRise of ISIS: A Threat We Can't IgnoreCalificación: 3.5 de 5 estrellas3.5/5 (137)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreCalificación: 4 de 5 estrellas4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Calificación: 4.5 de 5 estrellas4.5/5 (121)
- The Perks of Being a WallflowerDe EverandThe Perks of Being a WallflowerCalificación: 4.5 de 5 estrellas4.5/5 (2104)
- Her Body and Other Parties: StoriesDe EverandHer Body and Other Parties: StoriesCalificación: 4 de 5 estrellas4/5 (821)
- PEB Brochure PDFDocumento12 páginasPEB Brochure PDFBrian AbbottAún no hay calificaciones
- Drilling Fluid PDFDocumento23 páginasDrilling Fluid PDFcristinelbAún no hay calificaciones
- KDK College of Engineering, Nagpur Mechanical Department Session 2020-21Documento12 páginasKDK College of Engineering, Nagpur Mechanical Department Session 2020-21Chandani KannakeAún no hay calificaciones
- TN H01-Hand Book For Design of Steel StructuresDocumento210 páginasTN H01-Hand Book For Design of Steel StructuresEdward van Martino88% (8)
- Gek105060 File0060Documento12 páginasGek105060 File0060Mauricio GuanellaAún no hay calificaciones
- Lecture 9 - Natural Gas Processing NotesDocumento48 páginasLecture 9 - Natural Gas Processing NotesThomas FermatAún no hay calificaciones
- REPORT 2021 - CONSTRUCTION OF FLEXIBLE PAVEMENT BY USING PLASTICdemoDocumento25 páginasREPORT 2021 - CONSTRUCTION OF FLEXIBLE PAVEMENT BY USING PLASTICdemoAkash AhireAún no hay calificaciones
- DNA Transposons PDFDocumento14 páginasDNA Transposons PDFALAún no hay calificaciones
- Evaluation and Preparation of Guava Jam Stored at Ambient TemperatureDocumento10 páginasEvaluation and Preparation of Guava Jam Stored at Ambient Temperatureiftikhar AhmedAún no hay calificaciones
- Sta Apple (Chrysophyllum Cainito L.) : E. M. Ya Ia An F. G Tierrez-Rozco A To o o Su Ivers y Queretaro, MexicDocumento7 páginasSta Apple (Chrysophyllum Cainito L.) : E. M. Ya Ia An F. G Tierrez-Rozco A To o o Su Ivers y Queretaro, MexicHannah Mae SarzaAún no hay calificaciones
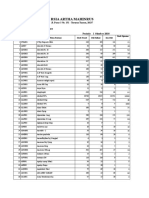
- Rsia Artha Mahinrus: Jl. Pasar 3 No. 151 - Terusan Tuasan, 20237Documento15 páginasRsia Artha Mahinrus: Jl. Pasar 3 No. 151 - Terusan Tuasan, 20237Rabyatul Maulida NasutionAún no hay calificaciones
- Selection of Pectin As Pharmaceutical Excepient On The Basis of Rheological BehaviorDocumento3 páginasSelection of Pectin As Pharmaceutical Excepient On The Basis of Rheological BehaviorВладимир КондратенкоAún no hay calificaciones
- Solder Wiki Inc Solder Melting PointsDocumento57 páginasSolder Wiki Inc Solder Melting PointsPJFAún no hay calificaciones
- DoymaDocumento28 páginasDoymaMariusAún no hay calificaciones
- Dynamic Shaft SealDocumento1 páginaDynamic Shaft SealSathishkumarAún no hay calificaciones
- DSU D InstallDocumento2 páginasDSU D InstallstarykAún no hay calificaciones
- Vessel Heads BottomDocumento9 páginasVessel Heads BottomFahad RockingAún no hay calificaciones
- Mndy ParchiDocumento858 páginasMndy ParchiPAN SERVICESAún no hay calificaciones
- Extraction and Purification of Astaxanthin From Shrimp Shells and TheDocumento6 páginasExtraction and Purification of Astaxanthin From Shrimp Shells and TheMarco CalixtoAún no hay calificaciones
- Influence of Test Equipment and Procedures On Obtained Accuracy in CPTUDocumento26 páginasInfluence of Test Equipment and Procedures On Obtained Accuracy in CPTUalistuguiAún no hay calificaciones
- Computing Liquid-Vapor Phase Diagrams For Non-Ideal Binary MixturesDocumento22 páginasComputing Liquid-Vapor Phase Diagrams For Non-Ideal Binary Mixturesmurdanetap957Aún no hay calificaciones
- Safety Data Sheet PropanDocumento9 páginasSafety Data Sheet PropanFahri SofianAún no hay calificaciones
- Microorganisms As Bio Indicators and BiosensorsDocumento42 páginasMicroorganisms As Bio Indicators and BiosensorsJAFFER YOUSUF85% (13)
- 1.4 Membrane Transport NotesDocumento13 páginas1.4 Membrane Transport Notesadri baigorriAún no hay calificaciones
- IIT Bombay Lab Manual Chemical EngineeringDocumento2 páginasIIT Bombay Lab Manual Chemical EngineeringAnuj SrivastavaAún no hay calificaciones
- S3D Settings PETG EsunDocumento4 páginasS3D Settings PETG Esunjose luis martinez martinezAún no hay calificaciones
- Doosan Mitsubishi 2.4L - Product Overview Training PDFDocumento105 páginasDoosan Mitsubishi 2.4L - Product Overview Training PDFkhairul100% (1)
- Hydrodesulfurization Unit For Natural Gas Condensate: Simulation Based On Aspen Plus SoftwareDocumento7 páginasHydrodesulfurization Unit For Natural Gas Condensate: Simulation Based On Aspen Plus SoftwareRuben MaciasAún no hay calificaciones
- Coring & Coring Analysis 2Documento21 páginasCoring & Coring Analysis 2Reband Azad100% (1)
- Ex6 Peroxide ValueDocumento7 páginasEx6 Peroxide ValueChidi IfenweobiAún no hay calificaciones