Documentos de Académico
Documentos de Profesional
Documentos de Cultura
Exam 1 Review
Cargado por
Alejandro GarciaDescripción original:
Derechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Exam 1 Review
Cargado por
Alejandro GarciaCopyright:
Formatos disponibles
Anatomy and Physiology II Fall 2011 Whisler
Lecture review materials for Exam 1
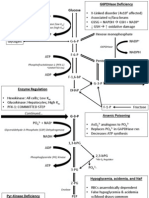
Topics covered on exam 1 Physiology as a science Biomolecules Carbohydrates Lipids Proteins Nucleic acids Chromosome structure and number in humans Cavities and body fluid compartments Protein structure and function Protein interactions Transcription and translation Cell membrane structure Energy and enzymes Carbonic acid equilibrium Synthetic pathways Cellular respiration pH
Chapter 1 1. Distinguish between anatomy and physiology. 2. Define homeostasis. Name some physiological parameters that are maintained homeostatically. 3. Across which body surfaces are materials exchanged with the external environment. Give examples of materials exchanged at each surface. 4. Give examples of many parameters that are under homeostatic control. 5. Give the normal range for pH of blood, stomach chyme, duodenum contents, and urine. 6. Give the normal body temperature in oF and oC. 7. What are the four key concepts (themes) in physiology? Summarize each. 8. Give several reasons why the results of scientific experiments using humans are often difficult to interpret. Words and roots to know.
Teleological Mechanistic Homeostasis Homeo Homo Extracellular Intracellular extra intra intro stasis dynamics parameter pathos pathological pathology pathophysiology compensation inter
Chapter 2 1. List the five important functional groups including name, shorthand and structure. 2. Explain the difference between saturated and unsaturated fatty acids. 3. List several chemicals built from cholesterol. 4. How is a trans fat related to an unsaturated fat? to a saturated fat? 5. To which biomolecule group do eicosanoids belong? To what chemical are they most structurally related? 6. What is the basic structure of an amino acid? How many amino acids commonly occur in human proteins? How many are essential? Define essential in this context.
7. List the names for the various size categories of amino acid/protein related molecules. 8. Describe the 4 levels of organization of proteins. 9. Compare and contrast fibrous and globular proteins both structurally and functionally. 10. What is a disulfide bond? Why is it important? 11. What types of forces hold proteins in tertiary and quaternary structures? 12. Define glycolipid, glycoprotein, lipoprotein. 13. List the three categories of nucleotide components. 14. What are the structural components of ATP? How does ATP differ from ADP? 15. Compare and contrast the structure of DNA and RNA. 16. Give many examples of protein function. 17. Write the equation for carbonic acid equilibrium. Based on this equation, how would you expect an increase in carbon dioxide in the blood to affect blood pH? How would you expect excess lactic acid production to affect respiration? 18. Explain, using a chemical equation, how the sodium bicarbonate in pancreatic and intestinal juice neutralizes stomach acid. 19. What characteristics permit proteins to do so many functions? 20. What changes occur as an enzyme (or other protein) binds to a substrate? 21. Under what circumstances might two ligands be competitors? 22. A drug that fits into the active site of an enzyme or membrane protein and causes the same reaction is called _____________? 23. What two effects can modulators have on proteins function? 24. Which modulators can either inhibit or activate proteins? 25. If a drug is an irreversible antagonist to oxygen on the hemoglobin molecule, what condition will result? 26. Allosteric inhibitors and activators both.. 28. An increase or decrease in cellular production of a protein to meet the needs of the cell is called ______________________ or ____________________________ 27. When excess ligand is present for an enzyme, what will happen to reaction rate? 28. What type of proteins can become saturated? End of chapter 2 questions related to basic chemistry review: 1-11; 24 list 1, 25 End of chapter 2 questions related to biomolecules and protein function 14-23, 27-32; 36 (do not calculate molecular weights), 40 End of chapter 2 questions related to pH and solutions 12-13; 24 list 2, 26; 34 Chapter 3 1) Distinguish between cavities and compartments. 2) Name the two major body compartments and describe the areas included within these compartments. 3) The extracellular compartment is further divided into what sub compartments? 4) If the phrase the three body compartments is used, which compartments are being referenced? 5) Distinguish between a cell membrane, a mucous membrane and a serous membrane. 6) Describe in detail the structure of a cell membrane including the location of all 4 major components. What are the major functions of each component? 7) Identify categories of membrane proteins based on position within the membrane. 8) What function may be performed by transmembrane proteins that cannot be performed by lipid anchored or peripheral proteins? 9) What are the three general types of cellular junctions? Note: you do not need to know the names of the specific proteins involved in cellular junctions. We will talk about cellular junctions later in the course as various types come up in context.
The remainder of Chapter 3 was thoroughly covered in A & P I . I expect you to be familiar with this material and although I will not test it explicitly on the exam, if you do not know it you will be handicapped in the course. Chapter 4. 2) Explain how concentration gradients can be used to do work. 3) Define and give examples of kinetic and potential energy conversions that occur in cells. 4) State the two laws of thermodynamics and give a biological example of each. 6) Given an example of a chemical reaction, identify it as a synthesis, decomposition, or exchange. Distinguish between single and double exchange (also called single and double displacement) reactions. 7) What are the main energy storage molecules in cells? Where specifically are these found in our bodies? 8) Relate synthesis and decomposition reactions to endergonic and exergonic processes. 9) Define activation energy. Graph examples of exergonic and endergonic reactions in terms of free energy over time. Be sure to label the axes, the reactants, products, initial state, final state, and activation energy. Which portion of each graph corresponds to net free energy change? Which corresponds to gross free energy change? 10) If B + C D is an endergonic reaction, what can you safely say about the reaction D B + C? 11) If ADP + P + R Q + S + ATP, what can you be sure is true about the energy change in the reaction of P + R Q + S ? Explain why your conclusion is correct. 12) How can you tell from the equation symbols whether a reaction is reversible? Sometimes the same enzyme catalyzes both the forward and reverse directions of the reaction. If the same enzyme can catalyze the reaction in either direction, what determines the direction of the net reaction? 13) Given an example of a coupled reaction, determine which is exergonic and which is endergonic based on which components are phosphorylated and which are dephosphorylated. 14) Recognize the oxidized and reduced forms of important energy carriers: NAD, NADH, FAD, FADH, 15) Define the term enzyme and give several specific examples of enzymes and functions in the human body. 16) Graph the effects of pH level and temperature change on enzyme function. Be able to interpret such a graph in terms of both pH and hydrogen ion concentration. 17) Distinguish between cofactors and coenzymes. How are vitamins related to coenzymes? 18) How does adding more substrate change the reaction rate of a reversible reaction? How does adding more product change the rate. 19) A typical reaction occurring in a certain cell type is: Q + ATP - Q-P + ADP At the present time, the following conditions exist in this cell: No available ATP, Plenty of reactant Q. a )Will this reaction proceed under current conditions? Explain why or why not. If it does proceed, what conditions could later stop it? b) If the conditions change to the following: lots of ATP present, 10 molecules of reactant Q present. Will the reaction proceed? Again, explain. 20) The law of mass action states for a reversible reaction, that the ratio of the reactants is equal to the ratio of the products at equilibrium. How is this idea different from saying that the amount of the reactants and products will be the same at equilibrium? 21) Consider the following equation: A +BC+D This reaction is initially in equilibrium in the cell. Later, a hormone triggers transport of more reactant A into the cell. How will the amounts of B, C and D change as a result of the increase in A? 22) Discuss the law of mass action using the carbonic acid equilibrium equation as an example.
23) What is being transferred during oxidation/reduction reactions? If the reactant is oxidized and the product is reduced, which has more energy when the reaction is complete? Besides energy loss or gain, how else has the product changed? 24) Be able to give/recognize many examples of dehydration synthesis/hydrolysis reactions. 25) What functional groups are often added, subtracted or exchanged during cellular chemical reactions? 26) If large substrate A is joined with large substrate B using energy from the breakdown of ATP, what type of reaction has occurred if the products are C + ADP + P. Think of as many terms as possible that would describe this reaction. Begin by writing out this question as a chemical reaction. 27) Discuss energy and functional group transfer during phosphorylation and dephosphorylation reactions. How is a dephosphorylation different from ligation if a large product is formed? 28) Recognize dehydration synthesis and hydrolysis reactions illustrated using the monomers of major macromolecules as examples. Explain which of these are energy storage molecules. 30) Distinguish between amination, deamination and transamination. Which must occur in order to extract energy from amino acids via glycolysis, the citric acid cycle and the e- transport chain? Which must occur to form an amino acid from an organic (non-nitrogen containing) acid? Which process might be used to convert one of the essential amino acids into a non-essential amino acid? 31) Define, metabolism, catabolism, anabolism. Distinguish between a one step chemical reaction and a metabolic pathway. What are the substrates called that make the central links in a metabolic pathway? 32) How can cells regulate metabolic pathways? 33) Explain feedback inhibition. Relate this process to competitive inhibition. 34) What are the 3 main pathways of the cellular respiration reaction? Where does each occur in the cell? 35) Where does the activation energy come from to initiate glycolysis (the glycolytic pathway)? 36) What are the products of glycolysis? 37) Given diagrams of the molecules mentioned, be able to evaluate changes in energy, phosphate groups and electrons during a chemical reaction. For example a) Explain in detail what is occurring when Fructose 6-phosphate is converted into fructose 1,6, bisphosphate during glycolysis. b) Explain in detail what is occurring when glyceraldehyde 3-phosphate is converted into 1,3, bisphosphoglycerate during glycolysis. 38) Which product of glycolysis continues on into the citric acid cycle? 39) When one carbon is removed from pyruvate, what products are formed? Where does this coupled reaction occur? 40) The acyl unit that connects to oxaloacetate in the citric acid cycle has how many carbons? How many carbon dioxide molecules will the citric acid cycle produce with each cycle? 41) What important energy carrying products are produced during the citric acid cycle? 42) Why is the citric acid cycle called a cycle? 43) Distinguish between substrate-level phosphorylation and oxidative phosphorylation. 44) Explain how the stored energy in NADH is used to form ATP during the electron transport chain. 45) Two products are formed by the complete breakdown of glucose by aerobic cellular respiration. Which product is formed during the electron transport chain? From what reactants is it formed? 46) Where do other major organic molecules that can be used for energy enter the cellular respiration pathway? Be specific. 47) Define each term: Which of the processes below are essentially the reverse of each other? Be sure to review Fig 22-2. Lipolysis Glycolysis Deamination beta oxidation Gluconeogenesis glycogenolysis fatty acid synthesis Chapter 4 review Q 1-19, 21-29, 30, 32
También podría gustarte
- Shoe Dog: A Memoir by the Creator of NikeDe EverandShoe Dog: A Memoir by the Creator of NikeCalificación: 4.5 de 5 estrellas4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeCalificación: 4 de 5 estrellas4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceCalificación: 4 de 5 estrellas4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)De EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Calificación: 4 de 5 estrellas4/5 (98)
- Grit: The Power of Passion and PerseveranceDe EverandGrit: The Power of Passion and PerseveranceCalificación: 4 de 5 estrellas4/5 (588)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe EverandThe Little Book of Hygge: Danish Secrets to Happy LivingCalificación: 3.5 de 5 estrellas3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerDe EverandThe Emperor of All Maladies: A Biography of CancerCalificación: 4.5 de 5 estrellas4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe EverandNever Split the Difference: Negotiating As If Your Life Depended On ItCalificación: 4.5 de 5 estrellas4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyCalificación: 3.5 de 5 estrellas3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealDe EverandOn Fire: The (Burning) Case for a Green New DealCalificación: 4 de 5 estrellas4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureCalificación: 4.5 de 5 estrellas4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryCalificación: 3.5 de 5 estrellas3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDe EverandTeam of Rivals: The Political Genius of Abraham LincolnCalificación: 4.5 de 5 estrellas4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaCalificación: 4.5 de 5 estrellas4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersCalificación: 4.5 de 5 estrellas4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaDe EverandThe Unwinding: An Inner History of the New AmericaCalificación: 4 de 5 estrellas4/5 (45)
- Rise of ISIS: A Threat We Can't IgnoreDe EverandRise of ISIS: A Threat We Can't IgnoreCalificación: 3.5 de 5 estrellas3.5/5 (137)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreCalificación: 4 de 5 estrellas4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Calificación: 4.5 de 5 estrellas4.5/5 (121)
- The Perks of Being a WallflowerDe EverandThe Perks of Being a WallflowerCalificación: 4.5 de 5 estrellas4.5/5 (2104)
- Her Body and Other Parties: StoriesDe EverandHer Body and Other Parties: StoriesCalificación: 4 de 5 estrellas4/5 (821)
- MCAT MetabolismDocumento4 páginasMCAT MetabolismNawledge9308100% (1)
- Arada Biology Model ExamDocumento14 páginasArada Biology Model ExamAbu ManAún no hay calificaciones
- Science: Modified Strategic Intervention MaterialsDocumento24 páginasScience: Modified Strategic Intervention MaterialsRoxanne Manaloto100% (1)
- Diagnostic TestDocumento2 páginasDiagnostic TestJOANA MARY VALLESERAún no hay calificaciones
- 2-Glycolysis 2014 Lecture - MDDocumento29 páginas2-Glycolysis 2014 Lecture - MDIbrahim BarhamAún no hay calificaciones
- Module 3 t1 2021Documento19 páginasModule 3 t1 2021erAún no hay calificaciones
- Chapter 22 Hypoxia 2017 Nunn S Applied Respiratory PhysiologyDocumento9 páginasChapter 22 Hypoxia 2017 Nunn S Applied Respiratory PhysiologyFontecha AnaAún no hay calificaciones
- Exercise PhysiologyDocumento51 páginasExercise PhysiologyFerisa paraswatiAún no hay calificaciones
- Final Exam (Practice) KEYDocumento11 páginasFinal Exam (Practice) KEYNevenKnezevicAún no hay calificaciones
- Life Sciences Grade 11 Exam Guidlines 2024Documento18 páginasLife Sciences Grade 11 Exam Guidlines 2024preciousrebotile133Aún no hay calificaciones
- Aerobic Metabolism of Lactic Acid BacteriaDocumento12 páginasAerobic Metabolism of Lactic Acid Bacteriaadel strikeAún no hay calificaciones
- The Effect of The Nature of Substrate On The Rate of Cellular Respiration in YeastDocumento9 páginasThe Effect of The Nature of Substrate On The Rate of Cellular Respiration in YeastAbe CeasarAún no hay calificaciones
- Eritrocite, Temp, ATPDocumento17 páginasEritrocite, Temp, ATPlucu8Aún no hay calificaciones
- 13 - Glycolysis TEAM438Documento28 páginas13 - Glycolysis TEAM438Haze MAún no hay calificaciones
- Segs-Chemistry of Life: Problem SetDocumento4 páginasSegs-Chemistry of Life: Problem SetMelindaSuitosColobong-GarpidaAún no hay calificaciones
- Copia de Mitochondria ColoringDocumento2 páginasCopia de Mitochondria ColoringDavid Justinico CastroAún no hay calificaciones
- 2009 Biology SyllabusDocumento44 páginas2009 Biology SyllabusImiosaAún no hay calificaciones
- Biochemistry (Metabolism) by Moses KDocumento405 páginasBiochemistry (Metabolism) by Moses Khaamajansi86% (7)
- Biological OxidationDocumento114 páginasBiological OxidationvanshikaAún no hay calificaciones
- Edexcel A2 Biology Unit 5 Revision Cards (Autosaved)Documento7 páginasEdexcel A2 Biology Unit 5 Revision Cards (Autosaved)nhmerali786100% (3)
- Introduction To BioenergeticsDocumento30 páginasIntroduction To BioenergeticsShahabAún no hay calificaciones
- Na Mlbio Ch10Documento28 páginasNa Mlbio Ch10itaemin243Aún no hay calificaciones
- HOPE 12 Q1 - Module-1 - Lesson-1-4Documento40 páginasHOPE 12 Q1 - Module-1 - Lesson-1-4Jhon FurioAún no hay calificaciones
- Hope - 3 Grade 12 Energy System: Quarter 1 Week 1 Module 1Documento5 páginasHope - 3 Grade 12 Energy System: Quarter 1 Week 1 Module 1Angelo Dela LlarteAún no hay calificaciones
- Sports Performance Measurement and Analytics - The Science of Assessing Performance, Predicting Future Outcomes, Interpreting Statistical Models, and ... Market Value of Athletes PDFDocumento321 páginasSports Performance Measurement and Analytics - The Science of Assessing Performance, Predicting Future Outcomes, Interpreting Statistical Models, and ... Market Value of Athletes PDFProf. Dr. Faris ShabbaAún no hay calificaciones
- Senior 12 Biology 1 Q2 - M6Documento18 páginasSenior 12 Biology 1 Q2 - M6Grandpa ZhongliAún no hay calificaciones
- Biology Quiz 2Documento3 páginasBiology Quiz 2Hafsa KhanAún no hay calificaciones
- Carbohydrate Complete Notes (B.pharm 2nd Sem)Documento25 páginasCarbohydrate Complete Notes (B.pharm 2nd Sem)DIPENDRA KUMAR KUSHAWAHAAún no hay calificaciones
- Cellular Respiration Webquest DBBBBDocumento5 páginasCellular Respiration Webquest DBBBB....Aún no hay calificaciones
- General ScienceDocumento147 páginasGeneral ScienceyabaeveAún no hay calificaciones