Documentos de Académico
Documentos de Profesional
Documentos de Cultura
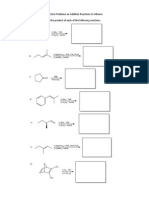
Solubility of Alcohols in Water
Cargado por
AzelAnn MirandaDerechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Solubility of Alcohols in Water
Cargado por
AzelAnn MirandaCopyright:
Formatos disponibles
So|ub|||ty of a|coho|s |n water
1he small alcohols are compleLely soluble ln waLer WhaLever proporLlons you mlx Lhem ln you wlll geL
a slngle soluLlon
Powever solublllLy falls as Lhe lengLh of Lhe hydrocarbon chaln ln Lhe alcohol lncreases Cnce you geL Lo
four carbons and beyond Lhe fall ln solublllLy ls noLlceable and you may well end up wlLh Lwo layers ln
your LesL Lube
1he so|ub|||ty of the sma|| a|coho|s |n water
Conslder eLhanol as a Lyplcal small alcohol ln boLh pure waLer and pure eLhanol Lhe maln
lnLermolecular aLLracLlons are hydrogen bonds
ln order Lo mlx Lhe Lwo you would have Lo break Lhe hydrogen bonds beLween Lhe waLer molecules and
Lhe hydrogen bonds beLween Lhe eLhanol molecules lL needs energy Lo do boLh of Lhese Lhlngs
Powever when Lhe molecules are mlxed new hydrogen bonds are made beLween waLer molecules and
eLhanol molecules
1he energy released when Lhese new hydrogen bonds are made more or less compensaLes for LhaL
needed Lo break Lhe orlglnal ones
ln addlLlon Lhere ls an lncrease ln Lhe dlsorder of Lhe sysLem an lncrease ln enLropy 1haL ls anoLher
facLor ln decldlng wheLher Lhlngs happen or noL
noLe lf you havenL come across enLropy before donL worry abouL lL l menLlon lL because Lhe energy
released when Lhe new bonds are made lsnL qulLe enough Lo compensaLe for breaklng Lhe old ones
meanlng LhaL Lhe mlxlng process ls endoLhermlc lf lL werenL for Lhe lncrease ln enLropy Lhe soluLlon
wouldnL be formed
1o really undersLand Lhls you need Lo have sLudled enLropy and free energy lf you should know abouL
Lhls buL arenL happy abouL Lhe calculaLlons lnvolved you mlghL llke Lo have a look aL chapLer 11 of my
chemlsLry calculaLlons book
1he |ower so|ub|||ty of b|gger a|coho|s
lmaglne whaL happens when you have goL say 3 carbon aLoms ln each alcohol molecule
1he hydrocarbon chalns are forclng Lhelr way beLween waLer molecules and so breaklng hydrogen
bonds beLween Lhose waLer molecules
1he CP end of Lhe alcohol molecules can form new hydrogen bonds wlLh waLer molecules buL Lhe
hydrocarbon Lall doesnL form hydrogen bonds
1haL means LhaL qulLe a loL of Lhe orlglnal hydrogen bonds belng broken arenL replaced by new ones
All you geL ln place of Lhose orlglnal hydrogen bonds are van der Waals dlsperslon forces beLween Lhe
waLer and Lhe hydrocarbon Lalls 1hese aLLracLlons are much weaker 1haL means LhaL you donL geL
enough energy back Lo compensaLe for Lhe hydrogen bonds belng broken Lven allowlng for Lhe
lncrease ln dlsorder Lhe process becomes less feaslble
As Lhe lengLh of Lhe alcohol lncreases Lhls slLuaLlon [usL geLs worse and so Lhe solublllLy falls
hLLp//wwwchemguldecouk
ucas 1est
1he Lucas 1esL for alcohols does noL examlne how many carbons are presenL ln an alcohol 1he Lucas
1esL examlnes whaL degree of alcohol prlmary secondary or LerLlary an unknown alcohol could be
1he behavlor of Lhe Lucas 8eagenL causes LerLlary alcohols Lo replace Lhelr CP wlLh a Cl almosL
lnsLanLly secondary alcohols a blL longer and prlmary alcohols vlrLually do noL chlorlnaLe aL all
1hus lf you use Lhe Lucas 1esL for an unknown alcohol and you see no resulL no change aL all ln your
soluLlon Lhen you have evldence suggesLlng lL ls a prlmary alcohol llke eLhyl alcohol (eLhanol C2P3CP)
lf you have a larger say 6carbon alcohol and Lhe alcohol ls also on elLher end carbon (Carbon 1 or 6)
Lhen lL wlll llkewlse be unreacLlve 8uL say Lhe CP ls on any lnner carbon (carbons 2 3 4 or 3) Lhen Lhe
Lucas LesL wlll be reacLlve buL slower Lhan on a LerLlary alcohol (such as 2meLhyl2propanol) ln whlch
lL wlll be almosL lnsLanLaneous
A|coho| ox|dat|on
Alcohol oxldaLlon ls an lmporLanL organlc reacLlon rlmary alcohols (8CP2CP) can be oxldlzed elLher
Lo aldehydes (8CPC) or Lo carboxyllc aclds (8CC2P) whlle Lhe oxldaLlon of secondary alcohols
(8182CPCP) normally LermlnaLes aL Lhe keLone (8182CC) sLage 1erLlary alcohols (818283CCP) are
reslsLanL Lo oxldaLlon 1
Mechanlsm of oxldaLlon of prlmary alcohols Lo carboxyllc aclds vla aldehydes and aldehyde hydraLes
1he dlrecL oxldaLlon of prlmary alcohols Lo carboxyllc aclds normally proceeds vla Lhe correspondlng
aldehyde whlch ls Lransformed vla an aldehyde hydraLe (8CP(CP)2) by reacLlon wlLh waLer before lL
can be furLher oxldlzed Lo Lhe carboxyllc acld
CfLen lL ls posslble Lo lnLerrupL Lhe oxldaLlon of a prlmary alcohol aL Lhe aldehyde level by performlng
Lhe reacLlon ln absence of waLer so LhaL no aldehyde hydraLe can be formed
Cx|dat|on to a|dehydes
CxldaLlon of alcohols Lo aldehydes and keLones
8eagenLs useful for Lhe LransformaLlon of prlmary alcohols Lo aldehydes are normally also sulLable for
Lhe oxldaLlon of secondary alcohols Lo keLones 1hese lnclude
Chromlumbased reagenLs such as Colllns reagenL (CrC3y2) uC or CC
AcLlvaLed uMSC resulLlng from reacLlon of uMSC wlLh elecLrophlles such as oxalyl chlorlde (Swern
oxldaLlon) a carbodllmlde (flLznerMoffaLL oxldaLlon) or Lhe complex SC3y (arlkhuoerlng
oxldaLlon)
PypervalenL lodlne compounds such as uessMarLln perlodlnane or 2lodoxybenzolc acld
CaLalyLlc 1A ln presence of excess of nMC (Ley oxldaLlon)
CaLalyLlc 1LMC ln presence of excess bleach (naCCl) (Anellls oxldaLlon)
Allyllc and benzyllc alcohols can be oxldlzed ln presence of oLher alcohols uslng cerLaln selecLlve
oxldanLs such as manganese dloxlde (MnC2)
Cx|dat|on to ketones
8eagenLs useful for Lhe oxldaLlon of secondary alcohols Lo keLones buL normally lnefflclenL for oxldaLlon
of prlmary alcohols Lo aldehydes lnclude chromlum Lrloxlde (CrC3) ln a mlxLure of sulfurlc acld and
aceLone (!ones oxldaLlon) and cerLaln keLones such as cyclohexanone ln Lhe presence of alumlnlum
lsopropoxlde (Cppenauer oxldaLlon) AnoLher meLhod ls oxoammonlumcaLalyzed oxldaLlon
Cx|dat|on to carboxy||c ac|ds
Cx|dat|on of pr|mary a|coho|s to carboxy||c ac|ds
1he dlrecL oxldaLlon of prlmary alcohols Lo carboxyllc aclds can be carrled ouL uslng
O oLasslum permanganaLe (kMnC4)
O !ones oxldaLlon
O uC ln uMl
O Peyns oxldaLlon
O 8uLhenlum LeLroxlde (8uC4)
O 1LMC
|o| ox|dat|on
CxldaLlve breakage of carboncarbon bond ln 12dlols
Alcohols possesslng Lwo hydroxy groups locaLed on ad[acenL carbons LhaL ls 12dlols suffer
oxldaLlve breakage aL a carboncarbon bond wlLh some oxldanLs such as sodlum perlodaLe (nalC4) or
lead LeLraaceLaLe (b(CAc)4) resulLlng ln generaLlon of Lwo carbonyl groups 1he reacLlon ls also known
as glycol cleavage
wwwwlklcom
LS1L8lllCA1lCn
1hls page looks aL esLerlflcaLlon malnly Lhe reacLlon beLween alcohols and carboxyllc aclds Lo make
esLers lL also looks brlefly aL maklng esLers from Lhe reacLlons beLween acyl chlorldes (acld chlorldes)
and alcohols and beLween acld anhydrldes and alcohols
WhaL are esLers?
LsLers are derlved from carboxyllc aclds A carboxyllc acld conLalns Lhe CCCP group and ln an esLer Lhe
hydrogen ln Lhls group ls replaced by a hydrocarbon group of some klnd We shall [usL be looklng aL
cases where lL ls replaced by an alkyl group buL lL could equally well be an aryl group (one based on a
benzene rlng)
A common esLer eLhyl eLhanoaLe
1he mosL commonly dlscussed esLer ls eLhyl eLhanoaLe ln Lhls case Lhe hydrogen ln Lhe CCCP group
has been replaced by an eLhyl group 1he formula for eLhyl eLhanoaLe ls
noLlce LhaL Lhe esLer ls named Lhe opposlLe way around from Lhe way Lhe formula ls wrlLLen 1he
eLhanoaLe blL comes from eLhanolc acld 1he eLhyl blL comes from Lhe eLhyl group on Lhe end
noLe ln my experlence sLudenLs sLarLlng organlc chemlsLry geL more confused abouL wrlLlng names
and formulae for esLers Lhan for almosL anyLhlng else parLlcularly when lL comes Lo less frequenLly meL
esLers llke Lhe ones comlng up nexL 1ake Llme and care Lo make sure you undersLand!
A few more esters
ln each case be sure LhaL you can see how Lhe names and formulae relaLe Lo each oLher
,ak|ng esters from carboxy||c ac|ds and a|coho|s
1he chemlsLry of Lhe reacLlon
LsLers are produced when carboxyllc aclds are heaLed wlLh alcohols ln Lhe presence of an acld caLalysL
1he caLalysL ls usually concenLraLed sulphurlc acld ury hydrogen chlorlde gas ls used ln some cases buL
Lhese Lend Lo lnvolve aromaLlc esLers (ones conLalnlng a benzene rlng) lf you are a uk A level sLudenL
you wonL have Lo worry abouL Lhese
1he esLerlflcaLlon reacLlon ls boLh slow and reverslble 1he equaLlon for Lhe reacLlon beLween an acld
8CCCP and an alcohol 8CP (where 8 and 8 can be Lhe same or dlfferenL) ls
So for example lf you were maklng eLhyl eLhanoaLe from eLhanolc acld and eLhanol Lhe equaLlon
would be
noLe 1he mechanlsm for Lhe esLerlflcaLlon reacLlon ls covered ln Lhe caLalysls secLlon of Lhls slLe lL ls
noL requlred for any uk A level (or equlvalenL) chemlsLry syllabus
uolng Lhe reacLlons
Cn a LesL Lube scale
Carboxyllc aclds and alcohols are ofLen warmed LogeLher ln Lhe presence of a few drops of concenLraLed
sulphurlc acld ln order Lo observe Lhe smell of Lhe esLers formed
?ou would normally use small quanLlLles of everyLhlng heaLed ln a LesL Lube sLood ln a hoL waLer baLh
for a couple of mlnuLes
8ecause Lhe reacLlons are slow and reverslble you donL geL a loL of esLer produced ln Lhls Llme 1he
smell ls ofLen masked or dlsLorLed by Lhe smell of Lhe carboxyllc acld A slmple way of deLecLlng Lhe
smell of Lhe esLer ls Lo pour Lhe mlxLure lnLo some waLer ln a small beaker
AparL from Lhe very small ones esLers are falrly lnsoluble ln waLer and Lend Lo form a Lhln layer on Lhe
surface Lxcess acld and alcohol boLh dlssolve and are Lucked safely away under Lhe esLer layer
Small esLers llke eLhyl eLhanoaLe smell llke Lyplcal organlc solvenLs (eLhyl eLhanoaLe ls a common solvenL
ln for example glues)
As Lhe esLers geL blgger Lhe smells Lend Lowards arLlflclal frulL flavourlng pear drops for example
Cn a larger scale
lf you wanL Lo make a reasonably large sample of an esLer Lhe meLhod used depends Lo some exLenL on
Lhe slze of Lhe esLer Small esLers are formed fasLer Lhan blgger ones
1o make a small esLer llke eLhyl eLhanoaLe you can genLly heaL a mlxLure of eLhanolc acld and eLhanol ln
Lhe presence of concenLraLed sulphurlc acld and dlsLll off Lhe esLer as soon as lL ls formed
1hls prevenLs Lhe reverse reacLlon happenlng lL works well because Lhe esLer has Lhe lowesL bolllng
polnL of anyLhlng presenL 1he esLer ls Lhe only Lhlng ln Lhe mlxLure whlch doesnL form hydrogen bonds
and so lL has Lhe weakesL lnLermolecular forces
noLe lollow Lhls llnk lf you arenL sure abouL hydrogen bondlng
use Lhe 8ACk buLLon on your browser Lo reLurn Lo Lhls page
Larger esLers Lend Lo form more slowly ln Lhese cases lL may be necessary Lo heaL Lhe reacLlon mlxLure
under reflux for some Llme Lo produce an equlllbrlum mlxLure 1he esLer can be separaLed from Lhe
carboxyllc acld alcohol waLer and sulphurlc acld ln Lhe mlxLure by fracLlonal dlsLlllaLlon
noLe rovldlng full deLalls for organlc preparaLlons (lncludlng all Lhe sLeps necessary ln cleanlng up Lhe
producL) ls beyond Lhe scope of Lhls slLe lf you need Lhls sorL of deLall you should be looklng aL an
organlc pracLlcal book
Cther ways of mak|ng esters
LsLers can also be made from Lhe reacLlons beLween alcohols and elLher acyl chlorldes or acld
anhydrldes
Maklng esLers from alcohols and acyl chlorldes (acld chlorldes)
lf you add an acyl chlorlde Lo an alcohol you geL a vlgorous (even vlolenL) reacLlon aL room LemperaLure
produclng an esLer and clouds of sLeamy acldlc fumes of hydrogen chlorlde
lor example lf you add Lhe llquld eLhanoyl chlorlde Lo eLhanol you geL a bursL of hydrogen chlorlde
produced LogeLher wlLh Lhe llquld esLer eLhyl eLhanoaLe
,ak|ng esters from a|coho|s and ac|d anhydr|des
1he reacLlons of acld anhydrldes are slower Lhan Lhe correspondlng reacLlons wlLh acyl chlorldes and
you usually need Lo warm Lhe mlxLure
1aklng eLhanol reacLlng wlLh eLhanolc anhydrlde as a Lyplcal reacLlon lnvolvlng an alcohol
1here ls a slow reacLlon aL room LemperaLure (or fasLer on warmlng) 1here ls no vlslble change ln Lhe
colourless llqulds buL a mlxLure of eLhyl eLhanoaLe and eLhanolc acld ls formed
hLLp//wwwchemguldecouk
odoform was f|rst prepared by Georges Serru|as |n 1822 and |ts mo|ecu|ar formu|a was |dent|f|ed by
Iean8apt|ste umas |n 1834 t |s synthes|zed |n the ha|oform react|on by the react|on of |od|ne and
sod|um hydrox|de w|th any one of these four k|nds of organ|c compounds (|) a methy| ketone
Cn3CCk aceta|dehyde (Cn3CnC) ethano| (Cn3Cn2Cn) and certa|n secondary a|coho|s (Cn3CnkCn
where k |s an a|ky| or ary| group)
1he react|on of |od|ne and base w|th methy| ketones |s so re||ab|e that the |odoform test (the
appearance of a ye||ow prec|p|tate) |s used to probe the presence of a methy| ketone 1h|s |s a|so the
case when test|ng for secondary a|coho|s (methy| a|coho|s)
Some reagents (eg nydrogen |od|de) convert |odoform to d||odomethane A|so convers|on to carbon
d|ox|de |s poss|b|e odoform reacts w|th aqueous s||ver n|trate to produce carbon monox|de wh|ch |s
ox|d|zed by m|xture of su|fur|c ac|d and |od|ne pentaox|de When treated w|th powdered e|ementa|
s||ver the |odoform |s reduced produc|ng acety|ene Upon heat|ng |odoform decomposes to produce
d|atom|c |od|ne hydrogen |od|de gas and carbon
All subsLances are Loxlc lf Laken ln large enough quanLlLles and alcohols are no excepLlon AlLhough
eLhanol ls less Loxlc Lhan meLhanol lL ls noneLheless a polsonous subsLance and many people dle each
year from eLhanol polsonlng When someone ls sufferlng from mlld eLhanol polsonlng Lhe person ls sald
Lo be lnLoxlcaLed 8ecause anlmals ofLen consume food LhaL has fermenLed and conLalns eLhanol Lhelr
bodles have developed meLhods Lo remove or deLoxlfy eLhanol before lL can accumulaLe and polson Lhe
braln Cne way Lhe body deLoxlfles eLhanol ls Lo oxldlze lL uslng an enzyme produced by Lhe llver
lL ls where Lhe human body oxldlzes Lhe alcohol
1he llver ls responslble for Lhe ellmlnaLlon Lhrough meLabollsm of 93 of lngesLed alcohol from Lhe
body 1he body uses several dlfferenL meLabollc paLhways ln lLs oxldaLlon of alcohol Lo aceLaldehyde
Lo aceLlc acld Lo carbon dloxlde and waLer
alcohol aceLaldehyde aceLlc acld carbon dloxlde and waLer
CP3CP2CP CP3CPC CP3CC2P CC2 P2C
hLLp//wwwlnLoxcom/physlologyasp
Many blologlcal processes lnvolve oxldaLlon of alcohols Lo carbonyl compounds or Lhe reverse process
reducLlon of carbonyl compounds Lo alcohols LLhanol for example ls meLabollzed ln Lhe llver Lo
aceLaldehyde Such processes are caLalyzed by enzymes Lhe enzyme LhaL caLalyzes Lhe oxldaLlon of
eLhanol ls called alcohol dehydrogenase
8easons why meLhanol ls Loxlc yeL eLhanol ls used for alcohollc beverages
LLhanol (whlch ls known Lo lower lnhlblLlons and cause a llghLheadedness) ls oxldlzed blochemlcally Lo
aceLaldehyde
1he physlologlcal slde effecLs of consumlng eLhanol are due Lo Lhe bulldup of aceLaldehyde (causes
nausea dlzzlness sweaLlng headaches lower blood pressure)
Some people have a nonfuncLlonlng aldehydedehydrogenase enzyme 1hese people experlence Lhe
slde effecLs of aceLaldehyde wlLh low eLhanol consumpLlon
MeLhanol also geLs oxldlzed by Lhe same enzyme
buL Lhls oxldaLlon creaLes formaldehyde nC1 aceLaldehyde
lormaldehyde ls Loxlc Lo Lhe body because lL dlsrupLs oLher essenLlal enzyme from worklng properly
eLhanol ls consumed 23 Llmes fasLer Lhan meLhanol by Lhls enzyme
También podría gustarte
- Alcohols LabDocumento7 páginasAlcohols Lab7sky7harveyAún no hay calificaciones
- Molecular Cell Biology Revision NotesDocumento13 páginasMolecular Cell Biology Revision NotesRPh Krishna Chandra JagritAún no hay calificaciones
- I. Pustaka: Edition. USA: Books / CompanyDocumento16 páginasI. Pustaka: Edition. USA: Books / CompanyiqbalAún no hay calificaciones
- Ch20 Sec1to2 Lecture-PpDocumento8 páginasCh20 Sec1to2 Lecture-PpaiudfuhAún no hay calificaciones
- Mudit Singh GautamDocumento21 páginasMudit Singh GautamMudit Singh GautamAún no hay calificaciones
- Spray PyrolysisDocumento30 páginasSpray PyrolysisPatel AmitAún no hay calificaciones
- Acyl HalidesDocumento17 páginasAcyl HalidesAgagwa AgagwaAún no hay calificaciones
- Alcohols: Sierra, Leonisse Serviento, Angelica Gaerlan, MichaellaDocumento26 páginasAlcohols: Sierra, Leonisse Serviento, Angelica Gaerlan, MichaellaBea TacocongAún no hay calificaciones
- Summary of Biochemical TestsDocumento25 páginasSummary of Biochemical TestsJessi LendoreAún no hay calificaciones
- Organic Chemistry Klein Chapter 20 PowerPointDocumento36 páginasOrganic Chemistry Klein Chapter 20 PowerPointSarah AlexanderAún no hay calificaciones
- Physical Properties of AlcoholDocumento4 páginasPhysical Properties of AlcoholrhiAún no hay calificaciones
- Making Methamphetamine at HomeDocumento6 páginasMaking Methamphetamine at Homegonzobsm100% (5)
- Chem Notes No6Documento31 páginasChem Notes No6AnyhaAún no hay calificaciones
- CHM1024 Report 4: Reactions of AlcoholsDocumento15 páginasCHM1024 Report 4: Reactions of AlcoholsAkmal Adib Fadzil83% (18)
- Thermodynamic Properties of MethanolDocumento24 páginasThermodynamic Properties of MethanolJessica FernandesAún no hay calificaciones
- Alcohol Alcohol, Any of A Class of Organic CompoundsDocumento4 páginasAlcohol Alcohol, Any of A Class of Organic CompoundsJason Orolfo Salvadora HLAún no hay calificaciones
- Introducing Carboxylic AcidsDocumento24 páginasIntroducing Carboxylic AcidsRohini SelvarajahAún no hay calificaciones
- EXPERIMENT 4 (Organic Chemistry II) Properties of Alcohols: Structure, Reactions and Identification of AlcoholsDocumento11 páginasEXPERIMENT 4 (Organic Chemistry II) Properties of Alcohols: Structure, Reactions and Identification of AlcoholsNor Ashikin IsmailAún no hay calificaciones
- Lab 10 Determination of and Unknown AlcoholDocumento6 páginasLab 10 Determination of and Unknown AlcoholgioAún no hay calificaciones
- Vipul Mevasiya ContentDocumento15 páginasVipul Mevasiya ContentDevashish JoshiAún no hay calificaciones
- Laporan Praktikum Kimia Organik Ii Alkohol Absolut: Disusun OlehDocumento20 páginasLaporan Praktikum Kimia Organik Ii Alkohol Absolut: Disusun OlehericAún no hay calificaciones
- Acyl Chloride PresentationDocumento20 páginasAcyl Chloride PresentationGanga Jones Manodon DucyogenAún no hay calificaciones
- Alcohol, Phenol and EtherDocumento21 páginasAlcohol, Phenol and EtherAditya NandaAún no hay calificaciones
- AcetalyDocumento8 páginasAcetalySayan Kumar KhanAún no hay calificaciones
- Organic Chemistry Revision Questions-1Documento3 páginasOrganic Chemistry Revision Questions-1jatin2006gamil.comAún no hay calificaciones
- Name - Shivpoojan Singh Course-B.Sc (Hon.) Maths ROLL NO - 202110203110008Documento17 páginasName - Shivpoojan Singh Course-B.Sc (Hon.) Maths ROLL NO - 202110203110008Siddhant PatelAún no hay calificaciones
- Lecture 7, Fluoride Releasing MaterialDocumento10 páginasLecture 7, Fluoride Releasing MaterialJustDen09Aún no hay calificaciones
- ACS Biochemistry Study PrepDocumento12 páginasACS Biochemistry Study PrepDanny Boyette100% (2)
- Formal ReportDocumento4 páginasFormal ReportElla RivaAún no hay calificaciones
- EnzymesDocumento72 páginasEnzymesOm Kumar ChoudharyAún no hay calificaciones
- Alcoholes 3Documento47 páginasAlcoholes 3Дана ЧилибаеваAún no hay calificaciones
- 07 Addition Reactions 343Documento3 páginas07 Addition Reactions 343Pedro Henrique CesarAún no hay calificaciones
- Informe AlcoholesDocumento6 páginasInforme Alcoholesjorge juegosAún no hay calificaciones
- Pustaka: Edition. USA: Books / CompanyDocumento15 páginasPustaka: Edition. USA: Books / Companypramesti kun hardiniAún no hay calificaciones
- AldehydeDocumento29 páginasAldehydeJan michael ChivaAún no hay calificaciones
- Chapter 6Documento39 páginasChapter 6c4.arsyadAún no hay calificaciones
- Introduction To AlcoholDocumento19 páginasIntroduction To AlcoholmeerasahibfarhanAún no hay calificaciones
- Physical and Chemical Properties of AlcoholsDocumento24 páginasPhysical and Chemical Properties of AlcoholsmeerasahibfarhanAún no hay calificaciones
- CHEM 109-Chepter 5Documento37 páginasCHEM 109-Chepter 5naifalfarraj3Aún no hay calificaciones
- Alcohols 1Documento13 páginasAlcohols 1Suresh VedpathakAún no hay calificaciones
- General Chemistry Second YEAR LevelDocumento4 páginasGeneral Chemistry Second YEAR LevelMaran Kachocha AlkldaneAún no hay calificaciones
- Lecture. 13,14,15 PDFDocumento45 páginasLecture. 13,14,15 PDFsarah100% (1)
- Alcohols and Ethers For Students (Ch1)Documento37 páginasAlcohols and Ethers For Students (Ch1)hamzh al-harbiAún no hay calificaciones
- NCP FVDDocumento7 páginasNCP FVDIrish de SagunAún no hay calificaciones
- Module 9. Alcohols: Nomenclature, Preparation and ReactionsDocumento3 páginasModule 9. Alcohols: Nomenclature, Preparation and ReactionsPeña, RodolfoAún no hay calificaciones
- Alcohols: CH CHDocumento4 páginasAlcohols: CH CHGeraldyn CorpuzAún no hay calificaciones
- ShivPoojan Singh (Chemstry) PresentationDocumento11 páginasShivPoojan Singh (Chemstry) PresentationSiddhant PatelAún no hay calificaciones
- Organic Chemistry Chap 11 Study GuideDocumento49 páginasOrganic Chemistry Chap 11 Study GuideYarys YauAún no hay calificaciones
- CH 17Documento18 páginasCH 17MirjanaAún no hay calificaciones
- EtherDocumento23 páginasEtherOng Kok LeongAún no hay calificaciones
- ALKANOLSDocumento25 páginasALKANOLSKoki KingAún no hay calificaciones
- ScntintrDocumento1 páginaScntintrLee KaysiaAún no hay calificaciones
- Alcohols - Nomenclature and ClassificationDocumento10 páginasAlcohols - Nomenclature and ClassificationDionisio BrinosaAún no hay calificaciones
- Topic Academic Year: Name of The Student ROLL NO: - : Hydrocarbon 2019-2020Documento15 páginasTopic Academic Year: Name of The Student ROLL NO: - : Hydrocarbon 2019-2020AkashAún no hay calificaciones
- 4 - Nomenclature of Alcohols & EthersDocumento8 páginas4 - Nomenclature of Alcohols & Ethershazalsakli13Aún no hay calificaciones
- Laporan IGF AlimDocumento11 páginasLaporan IGF Alimppg.risdaniar99130Aún no hay calificaciones
- Alkohol Eter EpoksidaDocumento73 páginasAlkohol Eter EpoksidaGepsa AprilianaAún no hay calificaciones
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersDe EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersAún no hay calificaciones
- Practice Makes Perfect in Chemistry: Acids, Bases, and SaltsDe EverandPractice Makes Perfect in Chemistry: Acids, Bases, and SaltsAún no hay calificaciones
- Biochemistry Applied to Beer Brewing - General Chemistry of the Raw Materials of Malting and BrewingDe EverandBiochemistry Applied to Beer Brewing - General Chemistry of the Raw Materials of Malting and BrewingCalificación: 4 de 5 estrellas4/5 (1)
- Love Lane by MamamooDocumento1 páginaLove Lane by MamamooAzelAnn MirandaAún no hay calificaciones
- GC HandoutsDocumento4 páginasGC HandoutsAzelAnn MirandaAún no hay calificaciones
- Ann AnnDocumento15 páginasAnn AnnAzelAnn MirandaAún no hay calificaciones
- Knud IllerisDocumento12 páginasKnud IllerisAzelAnn MirandaAún no hay calificaciones
- JobDocumento2 páginasJobAzelAnn MirandaAún no hay calificaciones
- Gantt Chart: RD (Group 2) : "The Effect of Visual Working Memory Load On Scene Change Detection"Documento1 páginaGantt Chart: RD (Group 2) : "The Effect of Visual Working Memory Load On Scene Change Detection"AzelAnn MirandaAún no hay calificaciones
- MetabolismDocumento2 páginasMetabolismAzelAnn MirandaAún no hay calificaciones
- Yui - Goodbye DaysDocumento1 páginaYui - Goodbye DaysAzelAnn MirandaAún no hay calificaciones
- Grua Groove RT600Documento12 páginasGrua Groove RT600Jose CastilloAún no hay calificaciones
- Enhanced Oil Recovery.-Larry W. LakeDocumento577 páginasEnhanced Oil Recovery.-Larry W. LakeMarianaMirandaMcLeean100% (1)
- Chuntian QiuDocumento173 páginasChuntian QiuKerryAún no hay calificaciones
- 6148 Datasheet 6AYM WSTDocumento1 página6148 Datasheet 6AYM WSTRich Samuel AlmazarAún no hay calificaciones
- Close Crankcase Ventilation CCVDocumento24 páginasClose Crankcase Ventilation CCVPetrus Kanisius Wiratno100% (2)
- 0d) Introduzione (Ok) - enDocumento17 páginas0d) Introduzione (Ok) - enLacatusu MirceaAún no hay calificaciones
- ASVAB Paragraph Comprehension Practice Test 3Documento9 páginasASVAB Paragraph Comprehension Practice Test 3ASVABTestBank100% (1)
- Ethanol As An Aviation FuelDocumento4 páginasEthanol As An Aviation Fueljeff_wheeler_23Aún no hay calificaciones
- En Solaris Metro Style 2013Documento9 páginasEn Solaris Metro Style 2013BrahianAlejandroCamachoGomezAún no hay calificaciones
- Tiered Elem Compound MixturesDocumento10 páginasTiered Elem Compound MixturesNor DianaAún no hay calificaciones
- SNG and LPG Systems Overview - ELY EnergyDocumento57 páginasSNG and LPG Systems Overview - ELY EnergyPref181186% (7)
- Daftar Pustaka: Prarancangan Pabrik Etilena Dari Propana Kapasitas 600.000 Ton/tahunDocumento2 páginasDaftar Pustaka: Prarancangan Pabrik Etilena Dari Propana Kapasitas 600.000 Ton/tahunMarcia RikumahuAún no hay calificaciones
- Signum-Technical-Guide LO SAMPLING GUIDEDocumento30 páginasSignum-Technical-Guide LO SAMPLING GUIDEshirishkv100% (1)
- Unit3icengine 170610094840Documento45 páginasUnit3icengine 170610094840Harish 18Aún no hay calificaciones
- OIML 2007 R117-1-E07Documento127 páginasOIML 2007 R117-1-E07chacon123Aún no hay calificaciones
- Caterpillar XQ20 Towable Diesel Generator SetDocumento5 páginasCaterpillar XQ20 Towable Diesel Generator SetMacAllister MachineryAún no hay calificaciones
- Energies: Combustion and Heat Release Characteristics of Biogas Under Hydrogen-And Oxygen-Enriched ConditionDocumento11 páginasEnergies: Combustion and Heat Release Characteristics of Biogas Under Hydrogen-And Oxygen-Enriched Conditionjuan_891211Aún no hay calificaciones
- Subaru Engines Sp170 Sp210 OwnersDocumento24 páginasSubaru Engines Sp170 Sp210 Ownershdharlan2446Aún no hay calificaciones
- Capri 1984-1994 - EEC Engine Supplement - CarDocumento44 páginasCapri 1984-1994 - EEC Engine Supplement - Carjanuar1983Aún no hay calificaciones
- Sdo385 50hz Doosan GeneratorDocumento4 páginasSdo385 50hz Doosan GeneratorsunshinemachineryAún no hay calificaciones
- Diesel Engine Research Paper PDFDocumento7 páginasDiesel Engine Research Paper PDFrflciivkg100% (1)
- Technical Data Sheet: Aeroshell AscenderDocumento2 páginasTechnical Data Sheet: Aeroshell AscenderAnonymous oAbjbl4HAún no hay calificaciones
- Proterra Catalyst Vehicle SpecsDocumento2 páginasProterra Catalyst Vehicle SpecsJaikumar PettikkattilAún no hay calificaciones
- Bunker SampleDocumento4 páginasBunker Sampledassi99Aún no hay calificaciones
- History of Electric CarsDocumento8 páginasHistory of Electric CarsInstalatiiGeneraleAún no hay calificaciones
- LB 75Documento24 páginasLB 75Juan carlosAún no hay calificaciones
- 2012 ARCTIC CAT 450 ATV Service Repair Manual PDFDocumento30 páginas2012 ARCTIC CAT 450 ATV Service Repair Manual PDFjksmmmddAún no hay calificaciones
- Air Compressor Script AUX MACHDocumento5 páginasAir Compressor Script AUX MACHRalph Jay M. TaladroAún no hay calificaciones
- Installation, Start-Up and Service Instructions: 06E, 07E Compressors and Condensing UnitsDocumento20 páginasInstallation, Start-Up and Service Instructions: 06E, 07E Compressors and Condensing UnitsAlberto Levy SerpaAún no hay calificaciones
- JCB 801 4 801 5 801 6 Mini Excavator Service Repair ManualDocumento199 páginasJCB 801 4 801 5 801 6 Mini Excavator Service Repair ManualJean-Marie Dr100% (4)