Documentos de Académico
Documentos de Profesional
Documentos de Cultura
ToxiRAE Correction Factors
Cargado por
DenisFerreiraCoutinho0 calificaciones0% encontró este documento útil (0 votos)
32 vistas3 páginasThis technical note provides guidelines for calculating correction factors for combustible gas (LEL) sensors. It discusses that LEL sensors require a minimum of 10% oxygen to function properly and are more sensitive to small molecules like methane than larger ones. Correction factors can be used to quantify different chemicals when calibrating with methane or pentane. The note provides a table of correction factors for various chemicals when calibrating with methane. It also discusses how sensitivity may change over time and recommends calibrating with the target gas or an alternative like propane or pentane if methane is not present.
Descripción original:
detec
Título original
ToxiRAE-Correction-Factors
Derechos de autor
© © All Rights Reserved
Formatos disponibles
PDF, TXT o lea en línea desde Scribd
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoThis technical note provides guidelines for calculating correction factors for combustible gas (LEL) sensors. It discusses that LEL sensors require a minimum of 10% oxygen to function properly and are more sensitive to small molecules like methane than larger ones. Correction factors can be used to quantify different chemicals when calibrating with methane or pentane. The note provides a table of correction factors for various chemicals when calibrating with methane. It also discusses how sensitivity may change over time and recommends calibrating with the target gas or an alternative like propane or pentane if methane is not present.
Copyright:
© All Rights Reserved
Formatos disponibles
Descargue como PDF, TXT o lea en línea desde Scribd
0 calificaciones0% encontró este documento útil (0 votos)
32 vistas3 páginasToxiRAE Correction Factors
Cargado por
DenisFerreiraCoutinhoThis technical note provides guidelines for calculating correction factors for combustible gas (LEL) sensors. It discusses that LEL sensors require a minimum of 10% oxygen to function properly and are more sensitive to small molecules like methane than larger ones. Correction factors can be used to quantify different chemicals when calibrating with methane or pentane. The note provides a table of correction factors for various chemicals when calibrating with methane. It also discusses how sensitivity may change over time and recommends calibrating with the target gas or an alternative like propane or pentane if methane is not present.
Copyright:
© All Rights Reserved
Formatos disponibles
Descargue como PDF, TXT o lea en línea desde Scribd
Está en la página 1de 3
Technical Note TN-156
CORRECTION FACTORS FOR COMBUSTIBLE GAS
(LEL) SENSORS
Note: This is a guideline document for calculating correction Oxygen Requirement and Matrix Effects
factors of LEL sensors. The user is encouraged to calibrate LEL sensors require oxygen for combustion and cannot be
the instrument with the target gas for most accurate used in environments that contain less than 10 vol% oxygen.
readings. Refer to the instrument settings and product This threshold is the safe limit for up to 100% LEL
manuals for information specific to each instrument. of nearly all chemicals, but it depends on the combustible
gas concentration. For example, for 10% LEL Methane, RAE
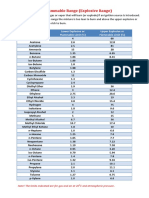
LEL Correction Factors
Systems LEL sensors show little or no oxygen dependence
RAE Systems LEL sensors can be used for the detection of a
down to about 5 vol% oxygen. Inserting an LEL sensor from
wide variety of combustible gases and vapors that exhibit
air into pure nitrogen can cause a transient response that
different responses. Because LEL sensors use a diffusion
decays after several minutes to the background reading. This
barrier to limit the gas flux to the catalytic bead, they tend to
is because the reference bead takes time to equilibrate with
have the greatest sensitivity to high-diffusivity compounds.
the slightly lower thermal conductivity of the nitrogen.
Therefore, they are substantially more sensitive to small
Likewise, other inert matrix gases may cause a transient
molecules like hydrogen and methane than to heavy
response.
components like kerosene. The best way to calibrate any
sensor to different compounds is to use a standard of the gas Humidity and temperature generally have little effect on the
of interest. However, Correction Factors (CFs) have been sensor response. Increasing temperature decreases the
determined that enable the user to quantify a large number of response by less than 4% between 0° and 40° C. Increasing
chemicals using only a single calibration gas, typically Methane RH (relative humidity) decreases the response by about 2%
or Pentane. In our LEL sensors, Correction Factors can be used between 20% and 90% RH.
in one of three ways:
Methane Sensitivity Changes
1. Calibrate the unit with Methane in the usual fashion to The Correction Factors in the table on the next page apply to
read in methane %LEL equivalents. Manually multiply new sensors. As a sensor becomes used and gradually loses
the reading by the Correction Factor (CF) to obtain the sensitivity, the response to Methane may decrease more
%LEL of the gas being measured. rapidly than for higher hydrocarbons. In this case, the
Correction Factors gradually decrease, and calibration with
2. Calibrate the unit with Methane and then call up the methane tends to overestimate the %LEL of the other gas.
Correction Factor from the instrument memory or choose Therefore, Methane calibration is the safest approach. RAE
the Correction Factor from the instrument memory first Systems LEL sensors do not exhibit changes in Correction
and then calibrate to methane. The unit after that reads Factors in laboratory tests, but may do so under special-use
directly in %LEL of the gas of interest. conditions. Calibrating with other organic vapors such as
Propane or Pentane is a good way to avoid Correction Factor
3. Calibrate the unit with Methane, but input an changes. The only drawback to this approach is that it is
equivalent, “corrected” span gas concentration when possible to underestimate Methane while still measuring the
prompted for this value. For example, to read in higher hydrocarbons. If Methane is known to be absent under
isopropanol LEL units, apply 20% LEL Methane but all circumstances, the use of propane or pentane calibration is
enter 20 x 2.6 = 52 for the span gas concentration. appropriate.
RAE Systems Inc. 877-723-2878 raesystems.com TN-156_06/13/PS 1
Technical Note TN-156
Correction Factors when Calibrating with
Non-methane Compounds
To obtain Correction Factors for other span gases, simply calculation is done internally in RAE Systems instruments that
divide the value on the methane scale in the table by the have separately selectable span and measurement gases.
Methane value for the span compound. For example, to obtain Therefore, in these cases, simply enter the span and
CFs on the n-pentane scale, divide all the numbers in the table, measurement compounds (without changing the CFs), and the
column LEL CF by 2.1. Thus, when calibrating with n-pentane unit automatically calculates and applies the new factor.
the new CF for ammonia is 1 /2.1 = 0.48. Note that this
100% Dimethylbutane 1.2 2.3
Chemical LEL CF*
LEL Dimethylpentane, 2,3- 1.1 2.5
(Vol%) Dioxane, 1,4- 2 2.4
Acetaldehyde 4 1.7 Ethane 3 1.4
Acetic acid 4 2.5 Ethanol 3.3 1.8
Acetic Anhydride 2.7 2.7 Ethene 2.7 1.3
Acetone 2.5 1.9 Ethyl acetate 2 2.4
Acetonitrile 3 1.7 Ethyl benzene 0.8 2.7
Acetylene 2.5 2.9 Ethyl bromide 6.8 2.6
Allyl Alcohol 2.5 2.1 Ethyl chloride 3.8 2
Ammonia 15 1 Ethyl ether 1.9 2.2
Aniline 1.3 6.3 Ethylamine 3.5 1.7
Benzene 1.2 2.1 Ethyl formate 2.8 2.2
Butadiene, 1,3- 2 1.8 Ethyl mercaptan 2.8 2
Butane, i- 1.8 1.7 Ethyl methyl ether 2 1.9
Butane, n- 1.9 1.9 Ethyl pentane 1.2 2.8
Butanol, i- 1.7 2.3 Ethylene oxide 3 1.7
Butanol, n- 1.4 2.8 Gasoline, 1.3 2.6
Butanol, t- 2.4 2.2 Heptane, n- 1.1 2.5
Butene-1 1.6 1.9 Hexadiene, 1,4- 2 2.3
Butene-2, cis 1.7 1.9 Hexane, n- 1.1 2.1
Butene-2, trans 1.8 1.9 Hydrazine 2.9 4.7
Butyric acid 2 3.7 Hydrogen 4 1
Carbon monoxide 12.5 1.3 Hydrogen cyanide 5.6 1.6
Carbonyl sulfide 12 1.9 Isobutene (Isobutylene) 1.8 1.6
Chlorobenzene 1.3 3.7 Isopropanol 2 2.2
Chloropropane, 1- 2.6 2.2 Methane 5 1
Cyanogen 6.6 1.8 Methanol 6 1.6
Cyclohexane 1.3 2.1 Methyl acetate 3.1 2.2
Cyclopropane 2.4 1.6 Methylamine 4.9 1.4
Decane, n- 0.8 3.3 Methyl bromide 10 2.4
Dichloroethane, 1,2- 6.2 5.4 Methyl chloride 8.1 1.8
Dichloromethane 13 2.3 Methyl ether 3.4 1.7
Diisobutyl ketone 0.8 3.2 Methyl ethyl ketone 1.4 2.2
Dimethyl sulfide 2.2 2 Methyl formate 4.5 1.9
RAE Systems Inc. 877-723-2878 raesystems.com 2
Technical Note TN-156
Methyl hexane 1.2 2.5 Propanol, n- 2.2 2.1
Methyl mercaptan 3.9 1.7 Propene 2 1.6
Methyl n-propyl ketone 1.5 2.4 Propyl ether, iso- 1.4 2.5
Methyl propionate 2.5 2.4 Propylamine, n- 2 1.9
Methylcyclohexane 1.2 2.5 Propylene oxide 2.3 1.9
Methylpentane 1.2 2.3 Propyne 1.7 1.6
Naphthalene 0.9 6.5 Toluene 1.1 2.4
Nitromethane 7.3 2.1 Triethylamine 1.2 2.5
Nonane, n- 0.8 3 Trimethylamine 2 1.9
Octane, n- 1 2.7 Trimethylbutane 1.2 2.5
Pentane, n- 1.5 2.1 Turpentine 0.8 3
Pentane, i- 1.4 1.9 Vinyl chloride 3.6 2
Pentane, Neo- 1.4 2.1 Xylene, m- 1.1 2.7
Pentene, 1- 1.5 2.1 Xylene, o- 0.9 2.8
Phosphine 1.6 1.5 Xylene, p- 1.1 2.9
Propane 2.1 1.4
* LEL CF values apply for MultiRAE families, ToxiRAE Pro and
other RAE Systems instruments. Values in bold type are
confirmed with RAE Systems Instruments. Others are calculated
from diffusion models for LEL sensors.
These correction factors are applicable for versions 1.64 for
ToxiRAE Pro and 1.16 for MultiRAE
Caution! Please refer to TN-144 for compounds that may
damage LEL sensors.
Note: Reported values of the lower explosive limit for jet fuels
range from about 0.3% to 0.9% by volume. An earlier third-
party test reported a CF for jet fuels of 3.35. However, we
have been unable to confirm this result and recommend using a
PID as a much more accurate method of measuring LEL for jet
and diesel fuels.
RAE Systems Inc. 877-723-2878 raesystems.com 3
También podría gustarte
- Chemesthesis: Chemical Touch in Food and EatingDe EverandChemesthesis: Chemical Touch in Food and EatingShane T. McDonaldAún no hay calificaciones
- Technical Note 156 Correction Factors For Combustible Gas LEL Sensorsnr 02 16Documento3 páginasTechnical Note 156 Correction Factors For Combustible Gas LEL Sensorsnr 02 16napoleon5976Aún no hay calificaciones
- C F C G (LEL) S: Orrection Actors For Ombustible AS EnsorsDocumento2 páginasC F C G (LEL) S: Orrection Actors For Ombustible AS EnsorsAli MehrpourAún no hay calificaciones
- Combustible Gas Sensors: Tech/App Note 7Documento3 páginasCombustible Gas Sensors: Tech/App Note 7Charles HuangAún no hay calificaciones
- Factor de Corrección Sensores DetectoresDocumento2 páginasFactor de Corrección Sensores DetectoresmanblancoferAún no hay calificaciones
- What Is %LEL / %UEL / Lower and Upper Explosive Limits For Flammable Gases and VaporsDocumento8 páginasWhat Is %LEL / %UEL / Lower and Upper Explosive Limits For Flammable Gases and VaporsSherwin Delfin CincoAún no hay calificaciones
- What Is LEL UEL PIDDocumento11 páginasWhat Is LEL UEL PIDHafed HafedAún no hay calificaciones
- What Is %LEL / %UEL /: Lower & Upper Explosive Limits For Flammable Gases & VaporsDocumento11 páginasWhat Is %LEL / %UEL /: Lower & Upper Explosive Limits For Flammable Gases & Vaporskenoly123Aún no hay calificaciones
- Envirotemp FR3 Fluid Dissolved Gas GuideDocumento27 páginasEnvirotemp FR3 Fluid Dissolved Gas GuideCarlos Gabriel Quintero RodríguezAún no hay calificaciones
- Acceptable and Dangerous Gas Levels in Confined SpacesDocumento6 páginasAcceptable and Dangerous Gas Levels in Confined SpacesQobil MirzaevAún no hay calificaciones
- GAS LEL and UELDocumento4 páginasGAS LEL and UELrajesh4dearsAún no hay calificaciones
- TP Flare Tricks RevDocumento8 páginasTP Flare Tricks Revtomjones77Aún no hay calificaciones
- Safety - What Is %LEL - %UEL and PID - Lower Explosive Limit (LEL) and The Upper Explosive Limit (UEL) - PhotoIonization DetectorDocumento4 páginasSafety - What Is %LEL - %UEL and PID - Lower Explosive Limit (LEL) and The Upper Explosive Limit (UEL) - PhotoIonization DetectorlavkeshAún no hay calificaciones
- Methane LEL, UEL.Documento4 páginasMethane LEL, UEL.Ovais FarooqAún no hay calificaciones
- Control Techniques For Gaseous Contaminants: 2.1 Gas Stream CharacteristicsDocumento22 páginasControl Techniques For Gaseous Contaminants: 2.1 Gas Stream CharacteristicsFelipe FonsecaAún no hay calificaciones
- Application Biomass Pyrolysis 990 Micro GC 5994 5581en AgilentDocumento3 páginasApplication Biomass Pyrolysis 990 Micro GC 5994 5581en AgilentEdinilson Ramos Camelo RamosAún no hay calificaciones
- Enthalpy of Combustion of AlcoholsDocumento8 páginasEnthalpy of Combustion of AlcoholsKian Paolo ManolesAún no hay calificaciones
- AOCS Method Ce 1c-89Documento4 páginasAOCS Method Ce 1c-89Lam Lai YanAún no hay calificaciones
- EtbeDocumento11 páginasEtbeSebastian Pala100% (1)
- Honeywell Gas Test Cell Quick StartDocumento9 páginasHoneywell Gas Test Cell Quick StartDan MarrAún no hay calificaciones
- Flare Gas Monitoring 1575295586Documento8 páginasFlare Gas Monitoring 1575295586Musab MohammedAún no hay calificaciones
- Manual Usuario Sensor Analox 9000 F1 Flammable SensorDocumento30 páginasManual Usuario Sensor Analox 9000 F1 Flammable SensorpevalpevalAún no hay calificaciones
- Name of Report: Production of Mythel Tertiary Butyl Ether (MTBE) From Methanol & ButyleneDocumento12 páginasName of Report: Production of Mythel Tertiary Butyl Ether (MTBE) From Methanol & Butyleneمصطفى سعدAún no hay calificaciones
- Specimen QPDocumento24 páginasSpecimen QPAnanda PillayAún no hay calificaciones
- Lower Explosive Limits of Combustible GasesDocumento1 páginaLower Explosive Limits of Combustible GasesSandro ChiliquingaAún no hay calificaciones
- Flashbacks: Causes and PreventionDocumento42 páginasFlashbacks: Causes and Preventiongivemore handizvihweAún no hay calificaciones
- ETBE Technical SheetDocumento28 páginasETBE Technical SheettoanvmpetrologxAún no hay calificaciones
- Decomposition of MtbeDocumento4 páginasDecomposition of MtbeEzzati AzizAún no hay calificaciones
- Park 2002Documento11 páginasPark 2002Juan Camilo AgudeloAún no hay calificaciones
- Gc955 Synspec Ozone Precursor Hydrocarbon: BackgroundDocumento4 páginasGc955 Synspec Ozone Precursor Hydrocarbon: BackgroundOmar SaaedAún no hay calificaciones
- K-Factor TechNoteDocumento5 páginasK-Factor TechNotetipcigromeroAún no hay calificaciones
- EthyleneDocumento8 páginasEthyleneEman El Dsouky100% (1)
- Silane Dilution ExhaustDocumento12 páginasSilane Dilution ExhaustArnelAún no hay calificaciones
- The Flammable Range (Explosive Range) : Gas Lower Explosive or Flammable Limit (%) Upper Explosive or Flammable Limit (%)Documento1 páginaThe Flammable Range (Explosive Range) : Gas Lower Explosive or Flammable Limit (%) Upper Explosive or Flammable Limit (%)Kaye Freyssinet Nermal AbangganAún no hay calificaciones
- LTC Condition AssessmentDocumento3 páginasLTC Condition AssessmentbertovalenAún no hay calificaciones
- Oxygenates and Paraffin, Olefin, Naphthene, Aromatic (O-PONA) Hydrocarbon Types in Low-Olefin Spark Ignition Engine Fuels by Gas ChromatographyDocumento8 páginasOxygenates and Paraffin, Olefin, Naphthene, Aromatic (O-PONA) Hydrocarbon Types in Low-Olefin Spark Ignition Engine Fuels by Gas Chromatography歐昱辰Aún no hay calificaciones
- ETBE ProductionDocumento6 páginasETBE ProductionS.P.Aún no hay calificaciones
- Composition of Crude OilDocumento9 páginasComposition of Crude OilDes C. BringelAún no hay calificaciones
- Articulo Poder CalorificoDocumento4 páginasArticulo Poder CalorificoPablo EspinosaAún no hay calificaciones
- Ethyl Acetate: - Technical Data SheetDocumento2 páginasEthyl Acetate: - Technical Data SheetFX Alan DarmasaputraAún no hay calificaciones
- LTC Diagnostics EpriDocumento10 páginasLTC Diagnostics Eprifahrian05Aún no hay calificaciones
- Gas Behavior and SeparationDocumento58 páginasGas Behavior and SeparationAhmed Sabry El-sotohyAún no hay calificaciones
- Paper Impurities'Effect Mitigation Case StudyDocumento14 páginasPaper Impurities'Effect Mitigation Case StudyHARVENDRA9022 SINGHAún no hay calificaciones
- Reservoir Fluids Day 10Documento142 páginasReservoir Fluids Day 10Bella cedricAún no hay calificaciones
- Reservoir Fluids Day 6Documento92 páginasReservoir Fluids Day 6Bella cedricAún no hay calificaciones
- Reservoir Fluids Day 2Documento31 páginasReservoir Fluids Day 2Bella cedricAún no hay calificaciones
- Reservoir Fluids Day 7Documento107 páginasReservoir Fluids Day 7Bella cedricAún no hay calificaciones
- Reservoir Fluids Day 4Documento57 páginasReservoir Fluids Day 4Bella cedricAún no hay calificaciones
- Photo Ionization DetectorDocumento25 páginasPhoto Ionization Detectorakash127Aún no hay calificaciones
- 76 1017 2.1 - K Factors PDFDocumento4 páginas76 1017 2.1 - K Factors PDFvkkamal100% (3)
- 2016, Error Due To Use of The AGA8 Equation of State Outside of Its Range of ValidityDocumento16 páginas2016, Error Due To Use of The AGA8 Equation of State Outside of Its Range of ValidityCO2 NIDFAún no hay calificaciones
- Nomograph LTC DgaDocumento0 páginasNomograph LTC DgaprevrtljivacAún no hay calificaciones
- Unit-1 Need For Altenate Fuel: AvailabilityDocumento5 páginasUnit-1 Need For Altenate Fuel: AvailabilitySyed MajeedAún no hay calificaciones
- Chapter 111 GT Tolalk Toluene Methylation To XylenesDocumento6 páginasChapter 111 GT Tolalk Toluene Methylation To Xylenesooimooi1111Aún no hay calificaciones
- Manning 1984 B3Documento25 páginasManning 1984 B3febrianirrAún no hay calificaciones
- Chapter 16 Eb X A Process For Ethylbenzene Separation and Xylenes UpgradeDocumento6 páginasChapter 16 Eb X A Process For Ethylbenzene Separation and Xylenes Upgradeooimooi1111Aún no hay calificaciones
- Flammability TestDocumento6 páginasFlammability TestHaithemAún no hay calificaciones
- Polarity IndexDocumento2 páginasPolarity Indexrnd labAún no hay calificaciones
- Tutorial 1Documento14 páginasTutorial 1Nurul AinaAún no hay calificaciones
- SLM 6 Grade 7 Science 1st Quarter Poster of Common Elements With Names Symbols and Uses 1Documento15 páginasSLM 6 Grade 7 Science 1st Quarter Poster of Common Elements With Names Symbols and Uses 1Rainier Kyle Moreno TulodAún no hay calificaciones
- CHM 361Documento16 páginasCHM 361Siti Maizatul Akma100% (2)
- R-2A Agar: Intended Use: CompositionDocumento3 páginasR-2A Agar: Intended Use: CompositionABDO ELJAAún no hay calificaciones
- IHC Methods 2009Documento63 páginasIHC Methods 2009romanciucclaudiaAún no hay calificaciones
- Portable Power - A Designer's Guide To Battery ManagementDocumento19 páginasPortable Power - A Designer's Guide To Battery ManagementMedSparkAún no hay calificaciones
- Includes Beryllium, Magnesium, Calcium, Strontium, Barium, and RadiumDocumento15 páginasIncludes Beryllium, Magnesium, Calcium, Strontium, Barium, and Radiumpink_music_loveAún no hay calificaciones
- Recycling of Plastics Using SolventDocumento65 páginasRecycling of Plastics Using SolventTeddy Ekubay GAún no hay calificaciones
- Z Potassium Industries 2007 2Documento17 páginasZ Potassium Industries 2007 2zhanvohrAún no hay calificaciones
- Exploits, Advances and Challenges Benefiting Beyond Li-Ion BatteryDocumento26 páginasExploits, Advances and Challenges Benefiting Beyond Li-Ion BatteryCaick Davila100% (1)
- Lab Report CHM 457 Exp 2Documento2 páginasLab Report CHM 457 Exp 2Irfan AzaharAún no hay calificaciones
- Chemistry Investigatory Project Class 12 Cold DrinkDocumento25 páginasChemistry Investigatory Project Class 12 Cold DrinkZara Hasan0% (1)
- Sandstone Reservoir Quality 2012 Stuart Haszeldine, University of EdinburghDocumento4 páginasSandstone Reservoir Quality 2012 Stuart Haszeldine, University of EdinburghLuciano J. MangueAún no hay calificaciones
- Nomenclature of A2Documento5 páginasNomenclature of A2SophieStevensAún no hay calificaciones
- A Clusters I 3Documento136 páginasA Clusters I 3Anuj KumarAún no hay calificaciones
- A To Z and Freeze ChemicalsDocumento65 páginasA To Z and Freeze ChemicalsdeendyalAún no hay calificaciones
- Optimization of Extracellular Keratinase Production by Aspergillus Terreus Isolated From Chicken's LitterDocumento7 páginasOptimization of Extracellular Keratinase Production by Aspergillus Terreus Isolated From Chicken's LitterKanhiya MahourAún no hay calificaciones
- Experiment-Salt Content in BrineDocumento2 páginasExperiment-Salt Content in BrineRyanAún no hay calificaciones
- CH 7 Practice Test Honor Chem Naming CompoundsDocumento8 páginasCH 7 Practice Test Honor Chem Naming CompoundsBeth0% (1)
- Cleaning and Sanitizing: at The End of This Module, Learners Should Be Able ToDocumento3 páginasCleaning and Sanitizing: at The End of This Module, Learners Should Be Able ToRovie Avenido Salindo100% (2)
- Agilent FPD Technical BulletinDocumento4 páginasAgilent FPD Technical Bulletinamittal111Aún no hay calificaciones
- Types of DisinfectantDocumento4 páginasTypes of DisinfectantGanjar AlaydrussaniAún no hay calificaciones
- Methods of Samrling and Test For Paints, VarnishesDocumento9 páginasMethods of Samrling and Test For Paints, Varnishessingaravelan narayanasamyAún no hay calificaciones
- Reaction Patent MelaminDocumento11 páginasReaction Patent MelaminAquae Tyo WijiantoAún no hay calificaciones
- Electrochemical BiosensorDocumento24 páginasElectrochemical BiosensorDyahrosa PranataAún no hay calificaciones
- BKCDocumento3 páginasBKCpavankumar9737Aún no hay calificaciones
- Saturated HydrocarbonDocumento60 páginasSaturated HydrocarbonMarvin K. Candor100% (1)
- Material Balances in The Production of Vinyl ChlorideDocumento5 páginasMaterial Balances in The Production of Vinyl ChlorideCHE.ENG1734Aún no hay calificaciones
- Bansal - ColoursDocumento3 páginasBansal - ColoursAniket SoodAún no hay calificaciones
- Tay Wei Peng D 2020 PHD ThesisDocumento257 páginasTay Wei Peng D 2020 PHD ThesisCamila FlorezAún no hay calificaciones
- C2-Organisation and Maintenance of The OrganismDocumento39 páginasC2-Organisation and Maintenance of The OrganismyourmoAún no hay calificaciones