Documentos de Académico
Documentos de Profesional
Documentos de Cultura
Wolpert, L. - 1994 - Do We Understand Development
Cargado por
contulmmivDerechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Wolpert, L. - 1994 - Do We Understand Development
Cargado por
contulmmivCopyright:
Formatos disponibles
.
28 OCTOBER 1994
571
Lewis Wolpert
Over the last 20 years, progress
in developmental biology has
been so dramatic that develop-
genes
are
gen-
erated between cells in the embryo that
then lead to spatial organization (pattern
formation), changes in form, and the generation of different cell types. Genes control
development by controlling cell behavior.
One can make a case that, given the eukaryotic cell, its elaboration to generate multicellular animals and plants was easy, compared to the evolution of the cell itself (1).
a
sense,
the
cell
is
more
complex
than the embryo, because the interactions
among cells in the embryo are much less
complex than interactions among the components of the cell. The response of an embryonic cell to a signal is very dependent
on the internal state of the cell, which in
turn depends on its developmental history.
In principle, most cell-to-cell signaling
could be achieved with a very small number of molecules, since the signals are always
selective rather than instructive. Indeed,
peptide growth factors are used as signals
repeatedly during development. Such
signals only select between a few possible new cell states; the complexity
of development lies in the internal
program of the cells.
It
is
surprising how few special
requires to understand
development. Fate maps, asymmetric
concepts one
division, induction, competence, positional information, determination,
and lateral inhibition will adequately
cover most systems. The real key to understanding development lies in cell biology, in the processes of signal transduction
and control of gene expression that result in
changes of cell state, movement, and growth.
Our best system for
formation
understanding has been a triumph
understood, and that the next 20
years will be devoted to filling in the
details. The most significant advances
have come from the application of molecular techniques and a greatly improved understanding of cell biology. So we
can begin to ask questions-like whether
the egg is computable.
In
pattern
of the combination of genetics and
biologists may be excused
for having the view, possibly an illusion, that the basic principles are
mental
During development, differences
control
is the fruit fly Drosophila (2). This
understanding
how
The author is in the Department of Anatomy and Developmental Biology, University College London, London, UK.
embryology. The two axes of the
organism, the anteroposterior and
dorsoventral, are initially independent of one another and are specified
by maternal gene products that form
gradients of positional information. After fertilization, the gradients activate a
cascade of zygotic genes, mainly coding for
protein transcription factors, and the embryo becomes divided into a number of regions, defined by the combination of the
activities of different genes. Along the
anteroposterior axis, a periodic pattern of
gene activity is established-the forerunner
of segments. Remarkably, each stripe of
gene activity is specified independently by
the local combination of proteins. Each
segment also acquires a unique identity
coded by the activity of a special set of
genes for transcription factors known as the
Hox genes (3).
Our understanding of early Drosophila
development has had a profound influence
on studies of other animal embryos with respect to approach, mechanism, and even
specific genes. Drosophila's importance as a
model for early development would be even
greater were it not that much patterning
occurs before the embryo becomes cellularized, and thus interaction between nuclei
is facilitated by the early and direct access
of transcription factors. By contrast, in
most other systems cell interactions require signal transduction across the cell
membrane.
Two model systems that have
vided an understanding at the
lecular level of how
promo-
small group of
cells are patterned are the fly eye
and the nematode vulva (4). In the
eye, a photoreceptor complex is
formed from eight cells, each with a
unique identity, and the proteins that
SCIENCE
VOL. 266
Downloaded from www.sciencemag.org on February 14, 2015
determine that identity-such as
sevenless and bride of sevenless-signal
only from one cell to its immediate neighbor. Again, in vulval development, the interactions are local. In both systems, signal
transduction involves the ras pathway.
Pattern formation in many animals with
larger numbers of cells is based on a mechanism where the cells first acquire a positional identity, which determines their future behavior. What is particularly exciting
and satisfying about pattern formation in
such systems is that similar principles, and
more striking, similar genes are involved in
diverse organisms-hydra, flies, worms, and
vertebrates. This is shown by the Hox
genes, which provide cells with positional
identity along the anteroposterior axis in
many animals (5). Also, one cannot but be
struck by, for example, the similarities between insect wing and chick wing development. In both systems it seems that there is
a signaling region that gives the cells their
positional identity and this region expresses
hedgehog-related genes, first identified by
their role in patterning segments in Drosophila (6).
Morphogenesis-change in form-also
relies on a rather restricted number of cellular activities. There is at the cellular level
a good understanding of the forces that, for
example, bring about gastrulation and can cause invagination and convergent extension (7). However, the
coordination, both biological and mechanical, of these
events and their link to gene
action still remains unclear.
What initiates, for example, cell movement in
gastrulation or neural crest
migration? Although one
can in principle envisage
mechanisms, the details are still lacking.
One obvious link between gene action and
morphogenesis is through the control of expression of cell adhesion molecules.
When one considers cell differentiation,
that is, the emergence of specific cell types,
it is hard to discern or even expect any general principles, other than that each cell
type seems to be specified by a combination
of transcription factors with varying degrees
of specificity. The association of the Myo-D
family with muscle differentiation may be
the exception rather than the rule (8).
In general then, it can be argued that
the principles of development are understood, although many crucial details at the
molecular level are missing. For example,
there is not a single case in all of vertebrate
development where an intercellular signal
has been unequivocally identified, even
though there are excellent candidates like
activin and fibroblast growth factor in early
amphibian development (9), and fibroblast
growth factor and retinoic acid in vertebrate limb development (10). We also still
do not know how signals are propagated
and whether there are, for example, graded
distributions of diffusible morphogens, even
though the evidence for graded properties
in positional identity is substantial (2, 11).
The downstream targets of the Hox genes
still remain elusive: How, for example, does
the change in just one gene change the an-
Do We Understand Development?
tenna of the fly into a leg? It may well be
that no general principles are involved in
the control of morphogenesis and cell differentiation. Even so, we do not yet have
an example where we understand in detail the development of a single adult
organ. We remain largely
ignorant of timing mechanisms and how the size of
different structures is controlled. It also has to be recognized that we do not yet know to
what extent the principles of animal development apply to plants, although recent
progress has been dramatic, and genes have
been identified that control the identity of
floral structures (12).
How many genes control development-as distinct from providing the
housekeeping functions of the cell? The answer is not known, but one can guess.
Analysis of early insect development suggests that only about 100 genes are involved in controlling patterning during
early development. And in the nematode
at least 50 genes are known that control
vulva development (13). If one thinks of,
say, 100 genes for each multicellular structure in the adult, then 50 different structures in Drosophila would require 5000
genes. For mammals, for which there are
some 350 distinct cell types, tens of thousands of genes might be needed. Understanding the function of so many genes is
made even more difficult by cases of apparent redundancy. That is, it is possible to
knock out certain genes in mice without
there being any obvious effect on the phenotype. It is likely that true redundancy is
illusory and merely reflects the failure to
provide the correct test for an altered phenotype. It may thus be very difficult to
work out the true function of such genes.
Will the egg be computable? That is,
given a total description of the fertilized
egg-the total DNA sequence and the location of all proteins and RNA-could one
predict how the embryo will develop? This
is a formidable task, for it implies that in
computing the embryo, it may be necessary
to compute the behavior of all the constituent cells. It may, however, be feasible if a
level of complexity of description of cell
behavior can be chosen that is adequate to
account for development but that does not
require each cell's detailed behavior to be
taken into account.
An analogy to some of these problems is
found in the analysis of protein folding,
which seems a much simpler problem, but
where
it may not be possible to
work
out
from an extensive database could provide
the best basis for making predictions about
development. It is not unreasonable to
think that enough will eventually be
known to program a computer and simulate some
aspects of development.
We will, however, understand much more
than we can predict. For
example, if a mutation
were introduced that altered
the structure of a single protein,
it is unlikely that it will be possible to predict its consequences.
So what will the next 20 years bring?
Undoubtedly powerful new techniques will
be invented that will enable us to understand the details of gene action and the
biochemistry and biophysics of cell behavior. Working out the detailed action of all
those genes, proteins, interactions, and kinases will be a hard slog and often tedious.
It is unlikely that any new general principles will be discovered. However, the current excitement will continue as we come
to understand the detailed mechanisms,
and as more and more similarities between
apparently different developmental systems
emerge. Almost certainly there will be new
ways of integrating particular aspects of development, and so we will learn, for example, the logic underlying the apparently
varied mechanisms for generating periodic
and the reasons for the variety of
mechanisms for setting up the axes in early
development. We can also look forward to
great progress in the area of evolution and
development. We may then see the solution to grand problems like how basic body
plans emerged, how they are conserved,
and the origin of developmental novelty.
We will thus come to understand how development constrains and directs the form
of all multicellular organisms.
structures
References
1.L. Wolpert, Biol. J. Linn. Soc. 39, 109 (1989); L.
Wolpert, Development(suppi.), in press.
2. P. Lawrence, The Making of a Fly (Blackwell, Ox-
ford, 1992).
3. ..and G. Morata, Cell 78, 181 (1994).
4. T. Grunwald and G. M. Rubin, ibid. 68, 271
(1992).
5. J. M. W. Slack, P. W. H. Holland, C. F. Graham,
Nature 361, 490 (1993); C. Kenyon, Cell 78, 175
(1994); R. Krumlauf, ibid., p. 191.
6. K. Basler and G. Struhl, Nature 368, 208 (1994);
R. D. Riddle, R. L. Johnson, E. Laufer, C. Tabin,
Cell 75, 1401 (1993).
7. R. E. Keller, J. Shih, C. Domingo, Development
(suppl.), p. 81 (1992).
8. E. N. Olsen, Genes Dev. 4,1454 (1991).
9. J. Slack, Curr. Biol. 4, 116 (1994).
10. L. Nieswander, S. Jeffreys, G. Martin, C. Tickle,
Nature, in press.
11. L. Wolpert, Development (suppl.), p. 3 (1989).
12. D. Weigel and E. M. Meyerowitz, Cell 78, 203
(1994).
13. H. R. Horvitz and P. W. Sternberg, Nature 351,
535 (1991).
14. C. Chothia, ibid. 357, 543 (1992).
Of Flies and Fishes
Christiane Nusslein-Volhard
vertebrates, the single most successful
approach for identifying genes of importance in development is based on the
surprising finding that important control
genes, or at least stretches of sequences
of control genes, are conserved through
evolution. Thus, for many genes discovered
in the invertebrate model systems Drosophila melanogaster and Caenorhabditis
elegans, "homologs" in frog, mouse, and
chicken have been identified, and their
functions in vertebrate organisms tested
with loss-of-function mutations made by
embryonic stem (ES) cell-mediated homologous recombination (1). Mouse genes
with similarity to selected Drosophila genes
frequently show severe loss-of-function
phenotypes. This result is in contrast to
what one generally finds for biochemically characterized vertebrate proteins,
In
the final structure from the sequence information by using first principles. Rather, the
solution will come from homology (14). As
with protein folding, homologies drawn
The author is at the Max-Planck-lnstitut fOr Entwicklungsbiologie, TObingen, Germany.
572
SCIENCE
VOL. 266
28 OCTOBER 1994
where in many instances the loss-of-function phenotype shows little or no visible
abnormality in the development or patterning of the animal.
Why do many Drosophila genes make a
fortune in vertebrate embryology? To answer this question, a brief review of the way
in which they were identified in Drosophila
is necessary. In flies, mutants were systematically sought; single genes essential for
embryonic pattern formation were identified by virtue of their loss-of-function
phenotype. Such saturation screens were
possible principally because Drosophila is
so ideal for genetic research. In particular,
the small number of chromosomes, and the
existence of giant chromosomes of the
salivary glands provided a unique physical
measure for the numbers of genes and the
analysis of chromosomal aberrations. Drosophila has about 6000 "essential" genes, of
which 5000 mutate to lethality (roughly
one-third each are embryonic, larval, or
También podría gustarte
- Medical Notes Clinical Medicine GuideDocumento257 páginasMedical Notes Clinical Medicine GuideMarina Andreou91% (22)
- Ebook Download LinksDocumento13 páginasEbook Download LinksPA201467% (3)
- Bottici Imaginal Politics 2011Documento17 páginasBottici Imaginal Politics 2011fantasmaAún no hay calificaciones
- Cardiac Physiology NotesDocumento11 páginasCardiac Physiology Notespunter11100% (1)
- Veena Das The Ground Between Anthropologists Engage Philosophy - Pgs. 288-314Documento27 páginasVeena Das The Ground Between Anthropologists Engage Philosophy - Pgs. 288-314Mariana OliveiraAún no hay calificaciones
- Meaning and Saying: Essays in the Philosophy of Language: Second EditionDe EverandMeaning and Saying: Essays in the Philosophy of Language: Second EditionAún no hay calificaciones
- (Univocal) Gilbert Simondon, Drew S. Burk-Two Lessons On Animal and Man-Univocal Publishing (2012)Documento89 páginas(Univocal) Gilbert Simondon, Drew S. Burk-Two Lessons On Animal and Man-Univocal Publishing (2012)Romero RameroAún no hay calificaciones
- German Idealism - ExistentialismDocumento71 páginasGerman Idealism - ExistentialismBeverlyn JamisonAún no hay calificaciones
- 2Q:Klwhkhdgdqg'Hohx) H7Kh3Urfhvvri0Dwhuldolw/: Configurations, Volume 13, Number 1, Winter 2005, Pp. 57-76 (Article)Documento21 páginas2Q:Klwhkhdgdqg'Hohx) H7Kh3Urfhvvri0Dwhuldolw/: Configurations, Volume 13, Number 1, Winter 2005, Pp. 57-76 (Article)Alex DeLargeAún no hay calificaciones
- Poultry Health ManagementDocumento12 páginasPoultry Health Managementhumanupgrade100% (1)
- Lecture 4: From The Linguistic Model To Semiotic Ecology: Structure and Indexicality in Pictures and in The Perceptual WorldDocumento122 páginasLecture 4: From The Linguistic Model To Semiotic Ecology: Structure and Indexicality in Pictures and in The Perceptual WorldStefano PernaAún no hay calificaciones
- Genius of The TinkererDocumento4 páginasGenius of The Tinkerershabir khanAún no hay calificaciones
- Eric Alliez-The Grace of The UniversalDocumento6 páginasEric Alliez-The Grace of The Universalm_y_tanatusAún no hay calificaciones
- FlapsDocumento27 páginasFlapsstegarescupavelAún no hay calificaciones
- Freud - Wolf Man Case Study (Demir Ayla Michelle PowerPoint) PDFDocumento24 páginasFreud - Wolf Man Case Study (Demir Ayla Michelle PowerPoint) PDFcontulmmivAún no hay calificaciones
- NCP - Ineffective Airway Clearance R/T Retained Secretions 2° BPNDocumento1 páginaNCP - Ineffective Airway Clearance R/T Retained Secretions 2° BPNCarl Elexer Cuyugan Ano100% (2)
- Logic of Phantasy 09Documento5 páginasLogic of Phantasy 09Chun-hsiung ChenAún no hay calificaciones
- NEGARESTANI, Reza - What Philosophy Does To The MindDocumento15 páginasNEGARESTANI, Reza - What Philosophy Does To The MindEze Iván DuarteAún no hay calificaciones
- Foucault On Discourse For Shakera GroupDocumento8 páginasFoucault On Discourse For Shakera Groupfarabi nawar100% (1)
- Schurmann and Singularity GranelDocumento14 páginasSchurmann and Singularity GranelmangsilvaAún no hay calificaciones
- BENNETT, David - Libidinal Economy, Prostitution and Consumer CultureDocumento30 páginasBENNETT, David - Libidinal Economy, Prostitution and Consumer CulturefbnfranciscoAún no hay calificaciones
- Gazzaniga - The New Cognitive NeurosciencesDocumento1434 páginasGazzaniga - The New Cognitive Neurosciencescontulmmiv89% (9)
- Neutral MonismDocumento59 páginasNeutral MonismValentin Matei100% (1)
- Sobre IgiturDocumento27 páginasSobre Igiturjosesecardi13Aún no hay calificaciones
- Bertalanffy Ensaio Sobre A Relatividade Das CategoriasDocumento22 páginasBertalanffy Ensaio Sobre A Relatividade Das CategoriasfabiophiloAún no hay calificaciones
- Cardiac ArrestDocumento30 páginasCardiac ArrestTommy NainggolanAún no hay calificaciones
- JL Nancy - The Creation of The World or GlobalizationDocumento137 páginasJL Nancy - The Creation of The World or Globalizationjquick4760Aún no hay calificaciones
- Carroll, S. B. - 2001 - Chance and Necessity. The Evolution of Morphological Complexity and DiversityDocumento8 páginasCarroll, S. B. - 2001 - Chance and Necessity. The Evolution of Morphological Complexity and Diversitycontulmmiv100% (1)
- Our Future Is Vegetal - 4 Michael MarderDocumento4 páginasOur Future Is Vegetal - 4 Michael MarderGalia Juárez VillegasAún no hay calificaciones
- Alexander Unzicker - The Mathematical Reality. Why Space and Time Are An IllusionDocumento217 páginasAlexander Unzicker - The Mathematical Reality. Why Space and Time Are An IllusioncontulmmivAún no hay calificaciones
- (Oxy) Med-Surg Checklist With RationaleDocumento13 páginas(Oxy) Med-Surg Checklist With RationaleUri Perez MontedeRamos100% (1)
- Waldenfels Time LagDocumento13 páginasWaldenfels Time LagnukiduzAún no hay calificaciones
- Transplanting the Metaphysical Organ: German Romanticism between Leibniz and MarxDe EverandTransplanting the Metaphysical Organ: German Romanticism between Leibniz and MarxAún no hay calificaciones
- Marc Faber - The Performance of Gold During Inflation and Deflation (2005.04.20)Documento20 páginasMarc Faber - The Performance of Gold During Inflation and Deflation (2005.04.20)contulmmivAún no hay calificaciones
- Cajori - Origins of Fourth Dimension ConceptsDocumento11 páginasCajori - Origins of Fourth Dimension ConceptsJan ČapekAún no hay calificaciones
- Ray Brassier - Deleveling. Against Flat OntologiesDocumento17 páginasRay Brassier - Deleveling. Against Flat OntologiesvoyameaAún no hay calificaciones
- Third Stage Complications and Post-Partum CollapseDocumento44 páginasThird Stage Complications and Post-Partum CollapseRamlah Ibrahim100% (1)
- Latour - What Is Given in ExperienceDocumento15 páginasLatour - What Is Given in ExperienceGiorgi ChubinidzeAún no hay calificaciones
- Alliez Rewritin TmodernityDocumento14 páginasAlliez Rewritin Tmodernitym_y_tanatusAún no hay calificaciones
- The Biomass Distribution On Earth (Yinon M. Bar-On, Rob Phillips, Ron Milo)Documento6 páginasThe Biomass Distribution On Earth (Yinon M. Bar-On, Rob Phillips, Ron Milo)delenda3Aún no hay calificaciones
- Blos, P. - 1963 - The Concept of Acting Out in Relation To The Adolescent ProcessDocumento19 páginasBlos, P. - 1963 - The Concept of Acting Out in Relation To The Adolescent ProcesscontulmmivAún no hay calificaciones
- Sloterdijk, Nerness and DaseinDocumento7 páginasSloterdijk, Nerness and DaseinAntonio CamposAún no hay calificaciones
- Sarah Kofman - Prometheus, The First PhilosopherDocumento11 páginasSarah Kofman - Prometheus, The First PhilosopherLouiseBundgaardAún no hay calificaciones
- Jean Petitot The - Morphodynamical - Turn - of - Cognitive - LinguisticsDocumento21 páginasJean Petitot The - Morphodynamical - Turn - of - Cognitive - LinguisticsArtaban Sedaghat100% (1)
- Ray Brassier - Concrete Rules and Abstract Machines-FinalDocumento31 páginasRay Brassier - Concrete Rules and Abstract Machines-FinalafsgAún no hay calificaciones
- Kiarina Kordela For SpecimenDocumento12 páginasKiarina Kordela For SpecimenMax FoxAún no hay calificaciones
- Mario Robles-Báez, On Marx's Dialectic of The Genesis of The Money Form PDFDocumento31 páginasMario Robles-Báez, On Marx's Dialectic of The Genesis of The Money Form PDFGriffin Bur100% (1)
- Xenos in PhiloctetesDocumento18 páginasXenos in PhiloctetesPhevos KorSimAún no hay calificaciones
- Whitebook On HegelDocumento8 páginasWhitebook On HegelLucas BalconiAún no hay calificaciones
- Andrew Cutrofello-Discipline and Critique Kant, Poststructuralism, and The Problem of Resistance-SUNY Press (1994)Documento180 páginasAndrew Cutrofello-Discipline and Critique Kant, Poststructuralism, and The Problem of Resistance-SUNY Press (1994)Elli DaskalakiAún no hay calificaciones
- Lacan On Foucault's Velasquez, TombrockelmanDocumento19 páginasLacan On Foucault's Velasquez, TombrockelmanGeorgy Plekhanov100% (1)
- Gustave Guillaume On ChronogenesisDocumento1 páginaGustave Guillaume On ChronogenesisNeamțu Alina-PaulaAún no hay calificaciones
- Bataille, Georges. Toward Real RevolutionDocumento11 páginasBataille, Georges. Toward Real Revolutionmarc.a.martins6667Aún no hay calificaciones
- Becoming Multitude: The AnomalistDocumento10 páginasBecoming Multitude: The AnomalistAlberto AltésAún no hay calificaciones
- Canguilhem Ideology & Rationality 1Documento18 páginasCanguilhem Ideology & Rationality 1edifyingAún no hay calificaciones
- Hegel and The Galilean Moons (4 Chapters)Documento16 páginasHegel and The Galilean Moons (4 Chapters)michael schroederAún no hay calificaciones
- Sadeq HedeyatDocumento20 páginasSadeq Hedeyatwax_the_van_arthurAún no hay calificaciones
- Hillis Miller Narrative and HistoryDocumento20 páginasHillis Miller Narrative and HistoryVasiliki PetsaAún no hay calificaciones
- Searle, John - Proper NamesDocumento9 páginasSearle, John - Proper NamesRobert ConwayAún no hay calificaciones
- Contemporary PluralismDocumento8 páginasContemporary PluralismTerence BlakeAún no hay calificaciones
- Ansell Pearson K. - Living The Eternal Return As The Event. Nietzsche With Deleuze 1997 ART ING 34Documento35 páginasAnsell Pearson K. - Living The Eternal Return As The Event. Nietzsche With Deleuze 1997 ART ING 34Geo BertazzoAún no hay calificaciones
- Masturbation Atonal World ZizekDocumento7 páginasMasturbation Atonal World ZizekSoren EvinsonAún no hay calificaciones
- Riggio-Legacy of Henri BergsonDocumento16 páginasRiggio-Legacy of Henri BergsonmikadikaAún no hay calificaciones
- Of Levinas and Shakespeare: "To See Another Thus"De EverandOf Levinas and Shakespeare: "To See Another Thus"Moshe GoldAún no hay calificaciones
- Granel, Who Comes After The SubjectDocumento6 páginasGranel, Who Comes After The Subjectkanebri100% (1)
- Michael Sukale (Auth.) - Comparative Studies in Phenomenology-Springer Netherlands (1977)Documento161 páginasMichael Sukale (Auth.) - Comparative Studies in Phenomenology-Springer Netherlands (1977)Juan PabloAún no hay calificaciones
- Myth of Everyday LifeDocumento22 páginasMyth of Everyday LifeAna-Marija SenfnerAún no hay calificaciones
- B, JDocumento155 páginasB, JGustavo NogueiraAún no hay calificaciones
- Moira Gatens Spinozas Disturbing Thesis Power Norms and Fiction in The Tractatus TheologicopoliticusDocumento14 páginasMoira Gatens Spinozas Disturbing Thesis Power Norms and Fiction in The Tractatus TheologicopoliticusRafael MuñizAún no hay calificaciones
- Laruelle (Pli 8)Documento11 páginasLaruelle (Pli 8)Ahmed RizkAún no hay calificaciones
- A Letter To E. E. Evans-Pritchard (L. Lévy-Bruhl)Documento8 páginasA Letter To E. E. Evans-Pritchard (L. Lévy-Bruhl)Eduardo Soares NunesAún no hay calificaciones
- Inheriting the Future: Legacies of Kant, Freud, and FlaubertDe EverandInheriting the Future: Legacies of Kant, Freud, and FlaubertAún no hay calificaciones
- Earth Day: Vision for Peace, Justice, and Earth Care: My Life and Thought at Age 96De EverandEarth Day: Vision for Peace, Justice, and Earth Care: My Life and Thought at Age 96Aún no hay calificaciones
- System of Nature by Baron D’Holbach 2 Volumes in One: Laws of the Physical World and of the Moral WorldDe EverandSystem of Nature by Baron D’Holbach 2 Volumes in One: Laws of the Physical World and of the Moral WorldAún no hay calificaciones
- Sea Level and Vertical Motion of Continents From Dynamic Earth Models Since The Late Cretaceous (2012)Documento28 páginasSea Level and Vertical Motion of Continents From Dynamic Earth Models Since The Late Cretaceous (2012)contulmmivAún no hay calificaciones
- Geodesy New 2022 DatumsDocumento38 páginasGeodesy New 2022 DatumscontulmmivAún no hay calificaciones
- Late Pleistocene and Holocene Dune Activity and Wind Regimes in The Western Sahara Desert of Mauritania (2002)Documento5 páginasLate Pleistocene and Holocene Dune Activity and Wind Regimes in The Western Sahara Desert of Mauritania (2002)contulmmivAún no hay calificaciones
- Geodesy and GravityDocumento304 páginasGeodesy and GravityplmplmplmplmplmAún no hay calificaciones
- Cenozoic Stratigraphy of The Sahara, Northern Africa (2009)Documento33 páginasCenozoic Stratigraphy of The Sahara, Northern Africa (2009)contulmmivAún no hay calificaciones
- Morner - 1980 - Paleogeoid Changes and Their Possible Impact On The Formation of Natural Resources in AfricaDocumento8 páginasMorner - 1980 - Paleogeoid Changes and Their Possible Impact On The Formation of Natural Resources in AfricacontulmmivAún no hay calificaciones
- McGucken, W. - 1978 - On Freedom and Planning in ScienceDocumento31 páginasMcGucken, W. - 1978 - On Freedom and Planning in SciencecontulmmivAún no hay calificaciones
- Ziman, J. - 1983 - The Collectivization of ScienceDocumento20 páginasZiman, J. - 1983 - The Collectivization of SciencecontulmmivAún no hay calificaciones
- Cenozoic Dynamics of The African Plate With Emphasis On The Africa-Eurasia Collision (1999) (ADN)Documento14 páginasCenozoic Dynamics of The African Plate With Emphasis On The Africa-Eurasia Collision (1999) (ADN)contulmmivAún no hay calificaciones
- Schulman, S., Rubinroit, C. I. - 1987 - The Second Individuation Process in Handicapped Adolescents PDFDocumento12 páginasSchulman, S., Rubinroit, C. I. - 1987 - The Second Individuation Process in Handicapped Adolescents PDFcontulmmivAún no hay calificaciones
- Marshall, D. L. - 2010 - The Origin and Character of Hannah Arendt's Theory of JudgmentDocumento27 páginasMarshall, D. L. - 2010 - The Origin and Character of Hannah Arendt's Theory of JudgmentcontulmmivAún no hay calificaciones
- Wolpert, L. - 1996 - Neural Development. Mysterious No More (Q)Documento2 páginasWolpert, L. - 1996 - Neural Development. Mysterious No More (Q)contulmmivAún no hay calificaciones
- Ziman, J. - 1983 - The Collectivization of ScienceDocumento20 páginasZiman, J. - 1983 - The Collectivization of SciencecontulmmivAún no hay calificaciones
- Carroll, S. B. - 2001 - Chance and Necessity. The Evolution of Morphological Complexity and Diversity PDFDocumento8 páginasCarroll, S. B. - 2001 - Chance and Necessity. The Evolution of Morphological Complexity and Diversity PDFcontulmmivAún no hay calificaciones
- Carroll, S. B. - 2001 - Chance and Necessity. The Evolution of Morphological Complexity and Diversity PDFDocumento8 páginasCarroll, S. B. - 2001 - Chance and Necessity. The Evolution of Morphological Complexity and Diversity PDFcontulmmivAún no hay calificaciones
- Wolpert, L. - 1994 - Do We Understand Development PDFDocumento2 páginasWolpert, L. - 1994 - Do We Understand Development PDFcontulmmivAún no hay calificaciones
- Rosen. R. - 1996 - On The Limitations of Scientific KnowledgeDocumento10 páginasRosen. R. - 1996 - On The Limitations of Scientific KnowledgecontulmmivAún no hay calificaciones
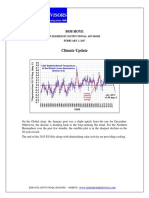
- 2017.02.04 Bob Hoye, Climate UpdateDocumento4 páginas2017.02.04 Bob Hoye, Climate UpdatecontulmmivAún no hay calificaciones
- McGucken, W. - 1978 - On Freedom and Planning in ScienceDocumento31 páginasMcGucken, W. - 1978 - On Freedom and Planning in SciencecontulmmivAún no hay calificaciones
- Refet S. Gürkaynak, Brian Sack, and Jonathan H. Wright - The TIPS Yield Curve and Inflation Compensation (2008.06) (Cf. Bloomberg FED5Y5Y Calculation Method)Documento42 páginasRefet S. Gürkaynak, Brian Sack, and Jonathan H. Wright - The TIPS Yield Curve and Inflation Compensation (2008.06) (Cf. Bloomberg FED5Y5Y Calculation Method)contulmmivAún no hay calificaciones
- David Brin - 1983 - The 'Great Silence'. The Controversy Concerning Extraterrestrial Intelligent LifeDocumento27 páginasDavid Brin - 1983 - The 'Great Silence'. The Controversy Concerning Extraterrestrial Intelligent LifecontulmmivAún no hay calificaciones
- Peak GoldDocumento30 páginasPeak GoldcontulmmivAún no hay calificaciones
- JeffersonDocumento353 páginasJeffersonAnarch777Aún no hay calificaciones
- Biology 1Documento15 páginasBiology 1maielsherif2020Aún no hay calificaciones
- Coombs and Gell ClassificationDocumento34 páginasCoombs and Gell ClassificationAnamAún no hay calificaciones
- Chapter 2. Acute and Chronic InflammationDocumento6 páginasChapter 2. Acute and Chronic InflammationWaseem Khan AfridiAún no hay calificaciones
- Urinary Tract Infection Nursing-Care-PlanDocumento3 páginasUrinary Tract Infection Nursing-Care-PlanRnspeakcomAún no hay calificaciones
- Chapter 2 BotanyDocumento6 páginasChapter 2 BotanyLebanan Aprille MarieAún no hay calificaciones
- DocDocumento4 páginasDocAnonymous eMOb79RNt5Aún no hay calificaciones
- 2012 Guidelines StableIschemicHeartDisease 11-20-12Documento121 páginas2012 Guidelines StableIschemicHeartDisease 11-20-12cephalicaAún no hay calificaciones
- RENAL QuestionDocumento15 páginasRENAL QuestionAnne CortezAún no hay calificaciones
- Blood Everywhere: A Case Study in BloodDocumento2 páginasBlood Everywhere: A Case Study in BloodAbdullahAún no hay calificaciones
- Njala University CHO BSC Level 200Documento6 páginasNjala University CHO BSC Level 200Mathew Moinina SonnieAún no hay calificaciones
- Nicolet VikingQuest PageDocumento2 páginasNicolet VikingQuest PageoviAún no hay calificaciones
- CirculatorySystem GizmoDocumento4 páginasCirculatorySystem GizmoNashly Ramirez50% (2)
- Department of Education: Republic of The PhilippinesDocumento2 páginasDepartment of Education: Republic of The PhilippinesDM Riel100% (1)
- 1st PUC Biology Nov 2017Documento2 páginas1st PUC Biology Nov 2017Abhi50% (2)
- Biology Exemplar ProblemsDocumento5 páginasBiology Exemplar ProblemsAkshatha NayakAún no hay calificaciones
- Wax and EstersDocumento7 páginasWax and EstersPamela Mae UcabAún no hay calificaciones
- Jace's PoV Manor SceneDocumento4 páginasJace's PoV Manor Sceneisa soaresAún no hay calificaciones
- Kinesiology-I: Introduction To Manual Muscle TestingDocumento12 páginasKinesiology-I: Introduction To Manual Muscle TestingKanwal KhanAún no hay calificaciones
- Chap 8Documento17 páginasChap 8Shane EsparasAún no hay calificaciones
- Red Blood Cell Disorders Part 1 and 2Documento7 páginasRed Blood Cell Disorders Part 1 and 2babael.zuher123Aún no hay calificaciones
- Immune System Organs and CellsDocumento30 páginasImmune System Organs and CellsRana AtefAún no hay calificaciones