Documentos de Académico
Documentos de Profesional
Documentos de Cultura
Exam
Cargado por
mohamed122Derechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Exam
Cargado por
mohamed122Copyright:
Formatos disponibles
3.
O12 - An Introduction to Reaction Stereoelectronics 2014-2015 OUTLINE ANSWERS
(a)
(i iii) Protonation of the substrate (synthesised by a Diels-Alder reaction) by formic
acid effects a Grob fragmentation. Re-drawing of this intermediate reveals that
the two stereogenic centres bearing methyl groups are carried through from the
starting material and map directly into the product. The decalin framework is
established by a vinologous Prins-type ring-closure which can give either the
axial or equatorial cationic isopropyl substituent depending on the relative
geometry of the interacting alkenes. The equatorial product predominates as
this is the thermodynamically preferred product. The cation is trapped out by
the formate anion and the methyl dienyl ether is hydrolysed to the enone in the
aqueous acidic conditions.
Problem solving. Grob fragmentations were discussed in lectures but not this
one. Envisaged mark schemes: 2 marks for correct arrow pushing for Grob
fragmentation; 2 marks for naming the stereoelectronic interactions; 2 marks for
indicating the anti-periplanar orbitals/bonds; 2 marks for correct arrow pushing for
remaining steps and 2 marks for indication of orbitals and co-planar arrangement of orbitals for the ring-closure.
(10 marks)
(b)
(i)
The order of events here is uncertain. The transformation could involve acid
catalysed intramolecular aldol condensation to give a decalin enone
intermediate in which the C-C double bond is aligned with the cyclopropane
such that facile fragmentation is possible to generate a tert-carbocation and
extended dienol (partly driven by relief of ring-strain).
Alternatively, protonation of the cyclohexanone could induce ring-opening of
the cyclopropane first, followed by an acid catalysed intramolecular aldol
condensation.
Either mechanism would attract full marks.
(ii)
Protonation of the endo hydroxyl group sets up a Wagner-Meerwein 1,2-shift
of the anti-periplanar C-C bond to yield a tertiary carbocation which undergoes
elimination to give the exo-methylene product. Direct E2 elimination on
protonation of the hydroxyl group is disfavoured because no protons have the
correct anti-periplanar orientation. The direction of the final elimination can
only take place in the manner shown as the alternative would lead to an antiBredt bridgehead alkene.
(iii)
Acid catalysed intramolecular aldol condensation (as for part ii, above) gives
an enone intermediate in which the C-C double bond is aligned with the
bridgehead C-C bond such that facile fragmentation is possible to generate a
tert-carbocation and extended dienol which becomes protonated at the position to regenerate an enone.
(iv)
Acid catalysed retro-aldol fragmentation driven by relief of strain in the
cyclobutane then tautomerism to give the isomeric enol which undergoes a 6exo-trig intramolecular aldol condensation.
Problem solving. Various rearrangements and fragmentations were discussed in
lectures but none of these ones. Envisaged mark schemes: 2 marks for correct arrow
pushing; 3 marks for indication of required anti-peripanar alignments and indication
of orbitals involved in the key rearrangements or fragmentations.
(5 marks each)
También podría gustarte
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeCalificación: 4 de 5 estrellas4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreCalificación: 4 de 5 estrellas4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe EverandNever Split the Difference: Negotiating As If Your Life Depended On ItCalificación: 4.5 de 5 estrellas4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceCalificación: 4 de 5 estrellas4/5 (894)
- Grit: The Power of Passion and PerseveranceDe EverandGrit: The Power of Passion and PerseveranceCalificación: 4 de 5 estrellas4/5 (587)
- Shoe Dog: A Memoir by the Creator of NikeDe EverandShoe Dog: A Memoir by the Creator of NikeCalificación: 4.5 de 5 estrellas4.5/5 (537)
- The Perks of Being a WallflowerDe EverandThe Perks of Being a WallflowerCalificación: 4.5 de 5 estrellas4.5/5 (2099)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureCalificación: 4.5 de 5 estrellas4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersCalificación: 4.5 de 5 estrellas4.5/5 (344)
- Her Body and Other Parties: StoriesDe EverandHer Body and Other Parties: StoriesCalificación: 4 de 5 estrellas4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Calificación: 4.5 de 5 estrellas4.5/5 (119)
- The Emperor of All Maladies: A Biography of CancerDe EverandThe Emperor of All Maladies: A Biography of CancerCalificación: 4.5 de 5 estrellas4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe EverandThe Little Book of Hygge: Danish Secrets to Happy LivingCalificación: 3.5 de 5 estrellas3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyCalificación: 3.5 de 5 estrellas3.5/5 (2219)
- The Yellow House: A Memoir (2019 National Book Award Winner)De EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Calificación: 4 de 5 estrellas4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaCalificación: 4.5 de 5 estrellas4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryCalificación: 3.5 de 5 estrellas3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDe EverandTeam of Rivals: The Political Genius of Abraham LincolnCalificación: 4.5 de 5 estrellas4.5/5 (234)
- Euro4 vehicle diesel engines 199 - 397 kW (270 - 540 hpDocumento6 páginasEuro4 vehicle diesel engines 199 - 397 kW (270 - 540 hpBranislava Savic63% (16)
- On Fire: The (Burning) Case for a Green New DealDe EverandOn Fire: The (Burning) Case for a Green New DealCalificación: 4 de 5 estrellas4/5 (73)
- The Unwinding: An Inner History of the New AmericaDe EverandThe Unwinding: An Inner History of the New AmericaCalificación: 4 de 5 estrellas4/5 (45)
- Palgrave Handbook of Research in Historical Culture and EducationDocumento847 páginasPalgrave Handbook of Research in Historical Culture and EducationGonzalo Garcia100% (1)
- Rise of ISIS: A Threat We Can't IgnoreDe EverandRise of ISIS: A Threat We Can't IgnoreCalificación: 3.5 de 5 estrellas3.5/5 (137)
- Little Book of Effective WritingDocumento44 páginasLittle Book of Effective Writingshalashvili100% (1)
- Summer Internship Project-NishantDocumento80 páginasSummer Internship Project-Nishantnishant singhAún no hay calificaciones
- Modern Analytic Chemistry 385Documento1 páginaModern Analytic Chemistry 385mohamed122Aún no hay calificaciones
- بحث oil analysis PDFDocumento77 páginasبحث oil analysis PDFmohamed122Aún no hay calificaciones
- Notes 5 HMR 3 Coupling 2Documento17 páginasNotes 5 HMR 3 Coupling 2Abdel Rahman MohamedAún no hay calificaciones
- RMN Coupling ConstantsDocumento38 páginasRMN Coupling ConstantsLarry AguirreAún no hay calificaciones
- بحث oil analysis PDFDocumento77 páginasبحث oil analysis PDFmohamed122Aún no hay calificaciones
- All en ADocumento5 páginasAll en AfatihAún no hay calificaciones
- En ASFA AU Koplik Atomic SpectrometryDocumento15 páginasEn ASFA AU Koplik Atomic SpectrometryRizka MaharanaAún no hay calificaciones
- Alkyl HalidesproblemDocumento1 páginaAlkyl Halidesproblemmohamed122Aún no hay calificaciones
- Exam OrganicDocumento1 páginaExam Organicmohamed122Aún no hay calificaciones
- Topic20 QuestionsDocumento3 páginasTopic20 Questionsmohamed122Aún no hay calificaciones
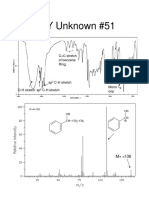
- KEY Unknown #51: C C Stretch of Benzene RingDocumento2 páginasKEY Unknown #51: C C Stretch of Benzene Ringmohamed122Aún no hay calificaciones
- BeerLaw PDFDocumento6 páginasBeerLaw PDFmohamed122Aún no hay calificaciones
- P.No Titles NO: Chapter Two - ChromatographyDocumento2 páginasP.No Titles NO: Chapter Two - Chromatographymohamed122Aún no hay calificaciones
- CH - 3Documento3 páginasCH - 3Phantom GamingAún no hay calificaciones
- E-banking and transaction conceptsDocumento17 páginasE-banking and transaction conceptssumedh narwadeAún no hay calificaciones
- 3.2 Probability DistributionDocumento38 páginas3.2 Probability Distributionyouservezeropurpose113Aún no hay calificaciones
- Merchandise Floor Ready Standards - Supplier InformationDocumento46 páginasMerchandise Floor Ready Standards - Supplier InformationGarmentLearner100% (1)
- Ilham Bahasa InggrisDocumento12 páginasIlham Bahasa Inggrisilhamwicaksono835Aún no hay calificaciones
- Technical File D13-MH, MG IMO Tier 11 GLDocumento18 páginasTechnical File D13-MH, MG IMO Tier 11 GLsfsdffdsdfsdfsdfAún no hay calificaciones
- The Bloodless GospelDocumento7 páginasThe Bloodless GospelKJVAún no hay calificaciones
- Mole Concept - DPP 09 (Of Lec 13) - Yakeen 2.0 2024 (Legend)Documento3 páginasMole Concept - DPP 09 (Of Lec 13) - Yakeen 2.0 2024 (Legend)Romeshchandra Class X-CAún no hay calificaciones
- Impact of Recruitment & Selection on Employee RetentionDocumento39 páginasImpact of Recruitment & Selection on Employee RetentiongizawAún no hay calificaciones
- Trimble Oem Gnss Bro Usl 0422Documento3 páginasTrimble Oem Gnss Bro Usl 0422rafaelAún no hay calificaciones
- Mesopotamia CivilizationDocumento56 páginasMesopotamia CivilizationYashika TharwaniAún no hay calificaciones
- Maximizing modular learning opportunities through innovation and collaborationDocumento2 páginasMaximizing modular learning opportunities through innovation and collaborationNIMFA SEPARAAún no hay calificaciones
- DANZIG, Richard, A Comment On The Jurisprudence of The Uniform Commercial Code, 1975 PDFDocumento17 páginasDANZIG, Richard, A Comment On The Jurisprudence of The Uniform Commercial Code, 1975 PDFandresabelrAún no hay calificaciones
- Dr. Malik's Farms BrochureDocumento18 páginasDr. Malik's Farms BrochureNeil AgshikarAún no hay calificaciones
- Week 6Documento7 páginasWeek 6Nguyễn HoàngAún no hay calificaciones
- Analysis of VariancesDocumento40 páginasAnalysis of VariancesSameer MalhotraAún no hay calificaciones
- Accidental PoisoningDocumento3 páginasAccidental PoisoningBRUELIN MELSHIA MAún no hay calificaciones
- WA Beretta M92FS Parts ListDocumento2 páginasWA Beretta M92FS Parts ListDenis Deki NehezAún no hay calificaciones
- LLM DissertationDocumento94 páginasLLM Dissertationjasminjajarefe100% (1)
- LGFL Service GuideDocumento24 páginasLGFL Service GuideThe Return of the NoiristaAún no hay calificaciones
- D257272 1200 FDD 002 R1 PDFDocumento420 páginasD257272 1200 FDD 002 R1 PDFTap Toan100% (1)
- Credit Risk Management Practice in Private Banks Case Study Bank of AbyssiniaDocumento85 páginasCredit Risk Management Practice in Private Banks Case Study Bank of AbyssiniaamogneAún no hay calificaciones
- Mama Leone's Profitability AnalysisDocumento6 páginasMama Leone's Profitability AnalysisLuc TranAún no hay calificaciones
- Turbine 1st Stage Nozzle - DPTDocumento15 páginasTurbine 1st Stage Nozzle - DPTAnonymous gWKgdUBAún no hay calificaciones
- Phys114 Ps 1Documento11 páginasPhys114 Ps 1Reine Amabel JarudaAún no hay calificaciones
- Migration (LIN 19/051: Specification of Occupations and Assessing Authorities) Instrument 2019Documento28 páginasMigration (LIN 19/051: Specification of Occupations and Assessing Authorities) Instrument 2019Ajay palAún no hay calificaciones