Documentos de Académico
Documentos de Profesional
Documentos de Cultura
New RP-HPLC Method For Simultaneous Estimation of Olmesartan Medoxomil and Hydrochlorothiazide in Combined Pharmaceutical Dosage Forms
Cargado por
IOSRjournalTítulo original
Derechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
New RP-HPLC Method For Simultaneous Estimation of Olmesartan Medoxomil and Hydrochlorothiazide in Combined Pharmaceutical Dosage Forms
Cargado por
IOSRjournalCopyright:
Formatos disponibles
IOSR Journal of Applied Chemistry (IOSR-JAC)
e-ISSN: 2278-5736.Volume 8, Issue 8 Ver. II (Aug. 2015), PP 106-109
www.iosrjournals.org
New RP-HPLC Method for Simultaneous Estimation of
Olmesartan Medoxomil and Hydrochlorothiazide in Combined
Pharmaceutical Dosage Forms
Jampala Balaji*1, T. Niranjan2, Bandi Ramachandra2 and N.V.S.Naidu1
1, 2
(Department of Chemistry, Sri Venkateswara University, Tirupathi, India-517502.)
Abstract: A simple RP-HPLC method developed for simultaneous estimation of Olmesartan medoxomil and
Hydrochlorothiazide in combined pharmaceutical dosage forms. This method validated as per ICH guidelines.
This method was simple, specific, precise, linear, accurate, robust and ruggedness for simultaneous analysis of
both Olmesartan medoxomil and Hydrochlorothiazide.
Keywords: Olmesartan medoxomil, Hydrochlorothiazide, RP-HPLC.
I.
Introduction
Olmesartan medoxomil is angiotensin II receptor blocker approved for use as an alternative therapeutic
anti-hypertensive agent. Chemically, it is (5-methyl-2-oxo-2H-1, 3-dioxol-4-yl) methyl, 4-(2-hydroxypropan-2yl)-2-propyl- 1-({4- [2- (2H- 1, 2, 3, 4-tetrazol -5-yl)phenyl]phenyl}methyl)-1H-imidazole-5-carboxylate.It is
shown in figure-1. It works by blocking a substance in the body that causes blood vessels to tighten. As a result,
olmesartan relaxes blood vessels. This lowers blood pressure and increases the supply of blood and oxygen to
the heart [1].
Hydrochlorothiazide is one of the oldest and widely used diuretics which is also used in the treatment
of hypertension [2]. Chemically, it is 6-chloro-1, 1-dioxo-3,4-dihydro-2H-1,2, 4-benzothiadiazine-7sulfonamide. It is shown in figure-2. Hydrochlorothiazide is a diuretic medication often used to treat high blood
pressure and swelling due to fluid buildup other uses include diabetes insipid us, renal tubular acidosis, and to
decrease the risk of kidney stones in those with high calcium level in the urine. For high blood pressure it is
often recommended as a first line treatment. It is taken by mouth and may be combined with other blood
pressure medications as a single pill to increase the effectiveness.
Several analytical HPLC methods have been found for the determination of individual and
simultaneous estimation of Olmesartan medoxomil and Hydrochlorothiazide [3]-[7]. So, the work has been done
to develop a simple, precise and accurate RP- HPLC method for simultaneous estimation of Olmesartan
medoxomil and Hydrochlorothiazide in pharmaceutical formulations.
(Figure 1: Olmesartan medoxomil)
(Figure 2: Hydrochlorothiazide)
DOI: 10.9790/5736-0882106109
www.iosrjournals.org
106 |Page
New RP-HPLC Method for Simultaneous Estimation of Olmesartan Medoxomil and
II.
Materials and Methods
Apparatus: HPLC (Model: Alliance 2695 separation module with UV-Visible detector, Make: Waters,
Software: Empower-2), Digital pH meter (Model: LP-1393, Make: POLMAN), Sonicator (Model: Soltec,
Make: Sonica), Electronic Weighing Balance (Model: ML204 Ia01, Make: Mettler Toledo).
Chemicals and Reagents: Acetonitrile (HPLC grade, Make: Qualigens fine chemicals), Sodium dihydrogen
orthophosphate (AR grade, Make: S.D Fine Chem. Ltd), Water (HPLC grade), Orthophosphoric acid (AR grade,
Make: Rankem), Olmesartan medoxomil (Richer Pharmaceuticals, Hyderabad, India), Hydrochlorothiazide
(Richer Pharmaceuticals, Hyderabad, India).
Chromatographic Conditions: This separation achieved by isocratically using Hypersil C18 column (150 X
4.6mm, 5m), column oven temperature 30 C, flow rate 1.0mL/min, UV at 254nm. Mobile phase was 0.1M
mono basic sodium phosphate buffer (pH: 3.0 0.1) adjusted with orthophosphoric acid and acetonitrile at
70:30 ratio. Diluent was water and acetonitrile in the ratio of 90:10(v/v). Run time was 10min.
Preparation of mobile phase: 13.8g of Sodium dihydrogen orthophosphate weighed and transferred in to
1000mL volumetric flask and make up to the mark with water and adjusted pH: 3.0(0.1)with diluted
Orthophosphoric acid solution. This buffer and acetonitrile were taken in the ratio of 70:30 and mixed well then
filtered through 0.45 m Millipore filter paper and sonicated using sonicator up to 15min.
Preparation of standard solution: Weighed 20mg of Olmesartan medoxomil and 12.5mg of
Hydrochlorothiazide working standards in 100mL volumetric flasks and dilute with 30mL of diluent and made
up to the mark with diluent for standards stock solution. Transferred 10mL of the standards stock solution in to
100mL volumetric flask and made up to the mark with diluent. This standards solution used to sample analysis.
Preparation of sample solution: 10 tablets of Olmesartan medoxomil and Hydrochlorothiazide powdered using
mortar. 200mg of this powder sample weighed and transferred in to 100ml volumetric flask and dissolved in
30mL of diluent then made up to the mark with diluent. This sample solution filtered then used as a sample
stock solution.10mL of this sample stock solution transferred in to 100mL volumetric flask and made up to the
mark with diluent. This solution is used for sample analysis.
Sample analysis: Injected 20L of blank (diluent), standard solution and sample solution in HPLC for sample
analysis. Retention time for Hydrochlorothiazide 1.99 and Retention time for Olmesartan medoxomil 4.93.
Method Validation
Specificity and Selectivity: The specificity of the method was checked by injecting blank solution and sample
solution. There was no interference from blank and excipients at the retention time of analytes peaks.
System suitability: Injected the system suitability solution and checked the system suitability. The tailing factor
value was less than 2.0, theoretical plates value was more than 2000 and resolution value was more than 2.0.
Linearity: Linearity was checked by preparing of five concentrations of the substance ranging from 25% to
150% level of the target. A concentration of 20ppm solution was proposed in the procedure as a 100% for
Olmesartan medoxomil, 12.5ppm solution was proposed in the procedure as a 100% for Hydrochlorothiazide.
Estimations were carried out as per the procedure mentioned. Observations were recorded and a linearity curve
was prepared using regression analysis. The correlation coefficient was 0.999 for Olmesartan medoxomil and
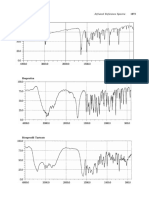
0.999 for Hydrochlorothiazide. Linearity graphs were shown in Figure 4 and Figure 5.
Accuracy: The accuracy of the method was determined by % of recovery method for the spiked concentration
levels of 50%, 100% and 150%. A concentration of 20ppm solution was proposed in the procedure as a 100%
for Olmesartan medoxomil, 12.5ppm solution was proposed in the procedure as a 100% for
Hydrochlorothiazide. The accuracy results were shown in table1 table 2.
Precision: The method precision and system precision were performed. The results were within limits for both
Olmesartan medoxomil and Hydrochlorothiazide. The results were shown in table3.
Robustness of the Method: Changing with the flow rate (0.1mL/minute) and column oven temperature (5C)
and calculated RSD. The results were within limits.
Ruggedness of the Method: The ruggedness of the method was performed with different analyst and different
day analysis and calculated RSD. The results were within limits.
LOD and LOQ: This method was having limit of detection value for Olmesartan medoxomil 1.2ppm, for
Hydrochlorothiazide 3.6ppm. The method was having limit of quantification value for Olmesartan medoxomil
3.0ppm, for Hydrochlorothiazide 9.0ppm.
III. Results And Discussions
The developed method was validated as per ICH guidelines for specificity, linearity, accuracy,
precision, robustness and ruggedness. The results were within limits.
DOI: 10.9790/5736-0882106109
www.iosrjournals.org
107 |Page
New RP-HPLC Method for Simultaneous Estimation of Olmesartan Medoxomil and
IV. Figures And Tables
(Figure 3: Chromatogram of Olmesartan Medoxomil and Hydrochlorothiazide)
(Figure 4: Linearity graph for Olmesartan Medoxomil)
(Figure 5: Linearity graph for Hydrochlorothiazide)
Concentration Level(spike)
50%
100%
150%
Recovery
100.97%
100.02%
100.71%
Table1: Accuracy for Olmesartan medoxomil
Concentration Level(spike)
50%
100%
150%
Recovery
101.78%
100.73%
99.42%
Table2: Accuracy for Hydrochlorothiazide
Precision
System Precision
Method Precision
Olmesartan medoxomil
0.34%
0.42%
Hydrochlorothiazide
0.28%
0.21%
Table3: Precision for Olmesartan medoxomil and Hydrochlorothiazide
DOI: 10.9790/5736-0882106109
www.iosrjournals.org
108 |Page
New RP-HPLC Method for Simultaneous Estimation of Olmesartan Medoxomil and
V.
Conclusion
The developed Reverse Phase HPLC method was very simple, selective and reproducible method for
simultaneous analysis of Olmesartan medoxomil and Hydrochlorothiazide in combined pharmaceutical dosage
forms. This method was having very low run time. It was accurate, precise, linear, robust and ruggedness
method. As per best of my knowledge this method was very simple method for simultaneous determination of
Olmesartan medoxomil and Hydrochlorothiazide in combined pharmaceutical dosage forms.
References
[1].
[2].
[3].
[4].
[5].
[6].
[7].
Napa Delhi Raj, Sockalingam Anbazhagan, Kunapareddy Anudeep Babu, Sunkara Narendra Babu, Chusena Narasimharaju
Bhimanadhuni, Validated stability indicating gradient RP-HPLC method for the estimation of antihypertensive drugs in bulk and
pharmaceutical dosage forms, International Current Pharmaceutical Journal, 1(11): 336-341,2012.
Suryadevara Vidyadhara, Reddyvalam Lankapalli CH Sasidhar, Ballipalli Venkateswara Rao, Koduri Tejaswi and Marupudi
Reshma, Method Development and Validation for Simultaneous Estimation of Olmesartan Medoxomil and Hydrochlorothiazide
by RP-HPLC, Oriental Journal of Chemistry, Vol. 30, No. (1):Pg. 195-201,2014.
Jain.P, Jain.A., Maliwal.D., Jain.V, Development and validation of spectrophotometric and RP-HPLC method for estimation of
olmesartan medoxomil in Tablet Dosage Form, International Journal of Pharma and Bio Sciences, 1:1-7. [DOI] PMid: 19819319,
2010.
Amudhavalli V, Lakshmi K. Karthick M, Determination of Olmesartan and Hydrochlorothiazide in pharmaceutical formulations
by RP-HPLC. Int J Chem Sci., 9: 470-476, 2011.
Narendra Devanaboyina, T.Satyanarayana and B.Ganga Rao, Simultaneous determination of Olmesartan and Hydrochlorothiazide
in Combined Pharmaceutical Dosage Form by RP-HPLC Method, International Journal of Pharma and Bio Sciences, Vol 3,Issue
2, 2012.
B. Raja and A. Lakshmana Rao, Development and Validation of Reversed Phase HPLC Method for Simultaneous Estimation of
Olmesartan and Hydrochlorothiazide in Combined Tablet Dosage Form, International Journal of Research in Pharmacy and
Chemistry, 1(3), 2011.
P. Ravisankar, J.Swathi, K.V.S.Santosh Kumar, P.Srinivasa Babu, Novel RP-HPLC Method for Simultaneous Determination of
Olmesartan Medoxomil, Amlodipine Besylate and Hydrochlorothiazide in Tablet Dosage Form, International Journal of
Biological & Pharmaceutical Research, 5(12): 927-936, 2014.
DOI: 10.9790/5736-0882106109
www.iosrjournals.org
109 |Page
También podría gustarte
- The Yellow House: A Memoir (2019 National Book Award Winner)De EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Calificación: 4 de 5 estrellas4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceCalificación: 4 de 5 estrellas4/5 (895)
- "I Am Not Gay Says A Gay Christian." A Qualitative Study On Beliefs and Prejudices of Christians Towards Homosexuality in ZimbabweDocumento5 páginas"I Am Not Gay Says A Gay Christian." A Qualitative Study On Beliefs and Prejudices of Christians Towards Homosexuality in ZimbabweInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- The Role of Extrovert and Introvert Personality in Second Language AcquisitionDocumento6 páginasThe Role of Extrovert and Introvert Personality in Second Language AcquisitionInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- The Impact of Technologies On Society: A ReviewDocumento5 páginasThe Impact of Technologies On Society: A ReviewInternational Organization of Scientific Research (IOSR)100% (1)
- A Review of Rural Local Government System in Zimbabwe From 1980 To 2014Documento15 páginasA Review of Rural Local Government System in Zimbabwe From 1980 To 2014International Organization of Scientific Research (IOSR)Aún no hay calificaciones
- A Proposed Framework On Working With Parents of Children With Special Needs in SingaporeDocumento7 páginasA Proposed Framework On Working With Parents of Children With Special Needs in SingaporeInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Attitude and Perceptions of University Students in Zimbabwe Towards HomosexualityDocumento5 páginasAttitude and Perceptions of University Students in Zimbabwe Towards HomosexualityInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Socio-Ethical Impact of Turkish Dramas On Educated Females of Gujranwala-PakistanDocumento7 páginasSocio-Ethical Impact of Turkish Dramas On Educated Females of Gujranwala-PakistanInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Assessment of The Implementation of Federal Character in Nigeria.Documento5 páginasAssessment of The Implementation of Federal Character in Nigeria.International Organization of Scientific Research (IOSR)Aún no hay calificaciones
- An Evaluation of Lowell's Poem "The Quaker Graveyard in Nantucket" As A Pastoral ElegyDocumento14 páginasAn Evaluation of Lowell's Poem "The Quaker Graveyard in Nantucket" As A Pastoral ElegyInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Relationship Between Social Support and Self-Esteem of Adolescent GirlsDocumento5 páginasRelationship Between Social Support and Self-Esteem of Adolescent GirlsInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Investigation of Unbelief and Faith in The Islam According To The Statement, Mr. Ahmed MoftizadehDocumento4 páginasInvestigation of Unbelief and Faith in The Islam According To The Statement, Mr. Ahmed MoftizadehInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Edward Albee and His Mother Characters: An Analysis of Selected PlaysDocumento5 páginasEdward Albee and His Mother Characters: An Analysis of Selected PlaysInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Comparative Visual Analysis of Symbolic and Illegible Indus Valley Script With Other LanguagesDocumento7 páginasComparative Visual Analysis of Symbolic and Illegible Indus Valley Script With Other LanguagesInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Topic: Using Wiki To Improve Students' Academic Writing in English Collaboratively: A Case Study On Undergraduate Students in BangladeshDocumento7 páginasTopic: Using Wiki To Improve Students' Academic Writing in English Collaboratively: A Case Study On Undergraduate Students in BangladeshInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Transforming People's Livelihoods Through Land Reform in A1 Resettlement Areas in Goromonzi District in ZimbabweDocumento9 páginasTransforming People's Livelihoods Through Land Reform in A1 Resettlement Areas in Goromonzi District in ZimbabweInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Importance of Mass Media in Communicating Health Messages: An AnalysisDocumento6 páginasImportance of Mass Media in Communicating Health Messages: An AnalysisInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Human Rights and Dalits: Different Strands in The DiscourseDocumento5 páginasHuman Rights and Dalits: Different Strands in The DiscourseInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Beowulf: A Folktale and History of Anglo-Saxon Life and CivilizationDocumento3 páginasBeowulf: A Folktale and History of Anglo-Saxon Life and CivilizationInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- An Exploration On The Relationship Among Learners' Autonomy, Language Learning Strategies and Big-Five Personality TraitsDocumento6 páginasAn Exploration On The Relationship Among Learners' Autonomy, Language Learning Strategies and Big-Five Personality TraitsInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- The Lute Against Doping in SportDocumento5 páginasThe Lute Against Doping in SportInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Role of Madarsa Education in Empowerment of Muslims in IndiaDocumento6 páginasRole of Madarsa Education in Empowerment of Muslims in IndiaInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Women Empowerment Through Open and Distance Learning in ZimbabweDocumento8 páginasWomen Empowerment Through Open and Distance Learning in ZimbabweInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Classical Malay's Anthropomorphemic Metaphors in Essay of Hikajat AbdullahDocumento9 páginasClassical Malay's Anthropomorphemic Metaphors in Essay of Hikajat AbdullahInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Substance Use and Abuse Among Offenders Under Probation Supervision in Limuru Probation Station, KenyaDocumento11 páginasSubstance Use and Abuse Among Offenders Under Probation Supervision in Limuru Probation Station, KenyaInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Kinesics, Haptics and Proxemics: Aspects of Non - Verbal CommunicationDocumento6 páginasKinesics, Haptics and Proxemics: Aspects of Non - Verbal CommunicationInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- A Study On The Television Programmes Popularity Among Chennai Urban WomenDocumento7 páginasA Study On The Television Programmes Popularity Among Chennai Urban WomenInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Designing of Indo-Western Garments Influenced From Different Indian Classical Dance CostumesDocumento5 páginasDesigning of Indo-Western Garments Influenced From Different Indian Classical Dance CostumesIOSRjournalAún no hay calificaciones
- Motivational Factors Influencing Littering in Harare's Central Business District (CBD), ZimbabweDocumento8 páginasMotivational Factors Influencing Littering in Harare's Central Business District (CBD), ZimbabweInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Micro Finance and Women - A Case Study of Villages Around Alibaug, District-Raigad, Maharashtra, IndiaDocumento3 páginasMicro Finance and Women - A Case Study of Villages Around Alibaug, District-Raigad, Maharashtra, IndiaInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- Design Management, A Business Tools' Package of Corporate Organizations: Bangladesh ContextDocumento6 páginasDesign Management, A Business Tools' Package of Corporate Organizations: Bangladesh ContextInternational Organization of Scientific Research (IOSR)Aún no hay calificaciones
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeCalificación: 4 de 5 estrellas4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe EverandThe Little Book of Hygge: Danish Secrets to Happy LivingCalificación: 3.5 de 5 estrellas3.5/5 (399)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaCalificación: 4.5 de 5 estrellas4.5/5 (266)
- Shoe Dog: A Memoir by the Creator of NikeDe EverandShoe Dog: A Memoir by the Creator of NikeCalificación: 4.5 de 5 estrellas4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureCalificación: 4.5 de 5 estrellas4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe EverandNever Split the Difference: Negotiating As If Your Life Depended On ItCalificación: 4.5 de 5 estrellas4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDe EverandGrit: The Power of Passion and PerseveranceCalificación: 4 de 5 estrellas4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryCalificación: 3.5 de 5 estrellas3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDe EverandThe Emperor of All Maladies: A Biography of CancerCalificación: 4.5 de 5 estrellas4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyCalificación: 3.5 de 5 estrellas3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealDe EverandOn Fire: The (Burning) Case for a Green New DealCalificación: 4 de 5 estrellas4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersCalificación: 4.5 de 5 estrellas4.5/5 (344)
- Rise of ISIS: A Threat We Can't IgnoreDe EverandRise of ISIS: A Threat We Can't IgnoreCalificación: 3.5 de 5 estrellas3.5/5 (137)
- Team of Rivals: The Political Genius of Abraham LincolnDe EverandTeam of Rivals: The Political Genius of Abraham LincolnCalificación: 4.5 de 5 estrellas4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaDe EverandThe Unwinding: An Inner History of the New AmericaCalificación: 4 de 5 estrellas4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreCalificación: 4 de 5 estrellas4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Calificación: 4.5 de 5 estrellas4.5/5 (121)
- The Perks of Being a WallflowerDe EverandThe Perks of Being a WallflowerCalificación: 4.5 de 5 estrellas4.5/5 (2102)
- Her Body and Other Parties: StoriesDe EverandHer Body and Other Parties: StoriesCalificación: 4 de 5 estrellas4/5 (821)
- Ayuspray ResearchDocumento9 páginasAyuspray ResearchJay AherkarAún no hay calificaciones
- 5 Proven Home Remedies For Seborrheic Dermatitis On The Scalp - Skin DroneDocumento7 páginas5 Proven Home Remedies For Seborrheic Dermatitis On The Scalp - Skin DroneIliasAún no hay calificaciones
- The Demise of Bloodletting: PaperDocumento6 páginasThe Demise of Bloodletting: PaperjjAún no hay calificaciones
- Biology Review Test - Part 2 - Grade 10Documento11 páginasBiology Review Test - Part 2 - Grade 10Arhant KarthikeyanAún no hay calificaciones
- 4 671945747968557164Documento67 páginas4 671945747968557164McMillanAún no hay calificaciones
- Category 3: Mammography (Digital) : 1260023072PL - Alpha - XLSX 1 of 15Documento15 páginasCategory 3: Mammography (Digital) : 1260023072PL - Alpha - XLSX 1 of 15Berkant Orçun ÇangalAún no hay calificaciones
- GuidelinesDocumento4 páginasGuidelinesChidambaram SeevakanAún no hay calificaciones
- FLACC Pain Assessment Scale 1 PDFDocumento1 páginaFLACC Pain Assessment Scale 1 PDFLucia NatashaAún no hay calificaciones
- Trismus Xerostomia and Nutrition Status PDFDocumento9 páginasTrismus Xerostomia and Nutrition Status PDFAstutikAún no hay calificaciones
- Magnified Healing BrochureDocumento3 páginasMagnified Healing BrochureMySecret Gardenmdp100% (1)
- Maastricht Interview For ChildrenDocumento14 páginasMaastricht Interview For ChildrenlillouanaAún no hay calificaciones
- The Last Sheet.: Ronnie D. Caringal Canubing National High SchoolDocumento57 páginasThe Last Sheet.: Ronnie D. Caringal Canubing National High SchoolVICKY HERMOSURAAún no hay calificaciones
- Softsoap Antibacterial Liquid Hand Soap Light MoisturizersDocumento12 páginasSoftsoap Antibacterial Liquid Hand Soap Light MoisturizersTim WillformAún no hay calificaciones
- Ibudilast: 1873 1873 JP Xvi Infrared Reference SpectraDocumento53 páginasIbudilast: 1873 1873 JP Xvi Infrared Reference SpectraDiego VargasAún no hay calificaciones
- Human Kinetics Web Resources PDFDocumento4 páginasHuman Kinetics Web Resources PDFyou waggaAún no hay calificaciones
- Proofed - Nurs 3020 Final EvaluationDocumento9 páginasProofed - Nurs 3020 Final Evaluationapi-313199824Aún no hay calificaciones
- Vitiligo - New Treatment ApproachDocumento7 páginasVitiligo - New Treatment Approachsawaira khanAún no hay calificaciones
- Nursing ExamDocumento8 páginasNursing ExamDa DungAún no hay calificaciones
- Munro 2007Documento17 páginasMunro 2007Jose RodriguezAún no hay calificaciones
- Soal Literasi Bahasa Inggris Dan PembahasannyaDocumento46 páginasSoal Literasi Bahasa Inggris Dan PembahasannyaSMP ADDARAENAún no hay calificaciones
- Vol14no6 PDF VersionDocumento143 páginasVol14no6 PDF VersionMark Reinhardt100% (1)
- Rcog PpromDocumento7 páginasRcog PpromDevi SyamAún no hay calificaciones
- Biocompactability Testing of Medical DevicesDocumento5 páginasBiocompactability Testing of Medical DevicesSathesh Kumar Annamalai100% (1)
- Tata Kelola DM Di FKTPDocumento29 páginasTata Kelola DM Di FKTPRSTN BoalemoAún no hay calificaciones
- Nursing Board Exam ReviewerDocumento32 páginasNursing Board Exam ReviewerRose Anne Mückl100% (9)
- WJCCM 11 33Documento8 páginasWJCCM 11 33medicshinobiAún no hay calificaciones
- Epidemiological Characteristics, Safety and Efficacy of Medical Cannabis inDocumento7 páginasEpidemiological Characteristics, Safety and Efficacy of Medical Cannabis inDanielAún no hay calificaciones
- Dermocosmetic Care For RosaceaDocumento16 páginasDermocosmetic Care For Rosaceariki fazar rahmaditaAún no hay calificaciones
- Premature Rupture of MembranesDocumento38 páginasPremature Rupture of MembranesArwa QishtaAún no hay calificaciones
- MCQ's With KEY GAYNAE - BDocumento7 páginasMCQ's With KEY GAYNAE - BSiraj Ul IslamAún no hay calificaciones