Documentos de Académico
Documentos de Profesional
Documentos de Cultura
Bonding Homework
Cargado por
Andrei FloreaDerechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Bonding Homework
Cargado por
Andrei FloreaCopyright:
Formatos disponibles
10.
3 Bonding Assessed Homework
Name: ..
Date Due: ..
AS Level Chemistry
80%
70%
60%
50%
40%
Below
1.3
%
Assessed Homework
Bonding
55
10.3 Bonding Assessed Homework
1.
(a)
An ammonium ion, made by the reaction between ammonia molecule and a
hydrogen ion, can be represented as shown in the diagram below.
+
H
H
(i)
H
H
Name the type of bond represented in the diagram N-H
.
(ii)
Name the type of bond represented in the diagram by NH
(iii)
In terms of electrons, explain why an arrow is used to represent this N H
bond
(iv)
In terms of electron pairs, explain why the bond angles in the NH 4+ ion are all
109o28
(7)
(b)
Define the term electronegativity
(2)
(c)
A bond between nitrogen and hydrogen can be represented as N - H
(i)
In this representation, what is the meaning of the symbol ?
10.3 Bonding Assessed Homework
(ii)
From this bond representation, what can be deduced about the electronegativity of
hydrogen relative to that of nitrogen?
(2)
(Total 11 marks)
2.
(a)
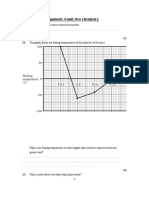
Both HF and HCl are molecules having a polar covalent bond. Their boiling points
are 293 K and 188 K respectively.
(i)
State which property of the atoms involved causes a bond to be polar.
...........................................................................................................................
...........................................................................................................................
(ii)
Explain, in terms of the intermolecular forces present in each compound,
why HF has a higher boiling point than HCl.
...........................................................................................................................
...........................................................................................................................
...........................................................................................................................
...........................................................................................................................
...........................................................................................................................
(4)
(b)
When aluminium chloride reacts with chloride ions, as shown by the equation
below, a co-ordinate bond is formed.
AlCl3 + Cl
AlCl4
Explain how this co-ordinate bond is formed.
..................................................................................................................................
.................................................................................................................................
.................................................................................................................................
(2)
10.3 Bonding Assessed Homework
(c)
+
Draw the shape of the PCl5 molecule and of the PCl4 ion. State the value(s) of
the bond angles.
+
PCl4
PCl5
Bond angle(s) ...................................... Bond angle(s)
..........................................
(4)
(Total 10 marks)
3.
Lithium hydride, LiH, is an ionic compound containing the hydride ion, H
The reaction between LiH and aluminium chloride, AlCl3, produces the ionic compound
LiAlH4
(a)
Balance the equation below which represents the reaction between LiH and AlCl3
LiH +
AlCl3
LiAlH4 +
LiCl
(1)
(b)
Give the electronic configuration of the hydride ion, H
......................
(1)
(c)
Predict the shape of the AlH 4 ion. Explain why it has this shape.
Shape ..........................................................................................................
Explanation .................................................................................................
......................
......................
(3)
(d)
A bond can be represented by H Al
Name this type of bond and explain how it is formed.
Type of bond ................................................................................................
Explanation ..
......................
(3)
(Total 8 marks)
10.3 Bonding Assessed Homework
4.
The table below shows some values of melting points and some heat energies needed for
melting.
Substance
I2
NaCl
HF
HCl
HI
Melting point/K
387
1074
190
158
222
Heat energy for melting /kJ mol1
7.9
28.9
3.9
2.0
2.9
(a)
Name three types of intermolecular forces
Force 1 ..
Force 2 ..
Force 3 ..
(3)
(b)
(i)
Describe the bonding in a crystal of iodine
..
(ii)
Name the crystal type which describes an iodine crystal
.
(iii)
Explain why heat energy is required to melt an iodine crystal.
(4)
(c)
In terms of intermolecular forces involved, suggest why
(i)
hydrogen fluoride requires more heat energy for melting than does of
hydrogen chloride
10.3 Bonding Assessed Homework
..
(ii)
Hydrogen iodide requires more heat energy for melting than does hydrogen
chloride.
..
(5)
(d)
(i)
Explain why the heat energy required to melt sodium chloride is large
(ii)
The heat energy need to vaporise one mole of sodium chloride (171 KJ mol1
) is much greater than the heat energy required to melt one mole of sodium
chloride. Explain why this is so.
..
(3)
(e)
In terms of structure and bonding, suggest why graphite has a very high melting and
boiling point
(2)
(Total 17 marks)
10.3 Bonding Assessed Homework
5.
The diagram below represents a section of a crystal of silicon dioxide.
Si
O
Si
Si
Si
O
(a)
O
O
Name an element which has a structure similar to this.
.
(b)
(1)
Name the type of bonding between silicon and oxygen in this crystal
.
(1)
(c)
Name the type of structure illustrated by this diagram
.
(1)
(d)
Describe the motion of the atoms in this crystalline solid
.
(2)
(e)
In terms of structure and bonding, describe what happens to the atoms in this
crystal when it melts
..
(f)
Explain why this crystal is a non conductor of electricity in the solid state and why
graphite is a good conductor.
10.3 Bonding Assessed Homework
(4)
(Total 9 marks)
También podría gustarte
- Arihant Class 9 Science Term 2 2 2021-2022 @NtseBookCornerDocumento110 páginasArihant Class 9 Science Term 2 2 2021-2022 @NtseBookCornerDishant Kumar73% (15)
- University of Cambridge Department of Chemical Engineering DatabookDocumento40 páginasUniversity of Cambridge Department of Chemical Engineering DatabookwaterdrinkAún no hay calificaciones
- Topic 09.3 Group IVDocumento3 páginasTopic 09.3 Group IVzafarchem_iqbalAún no hay calificaciones
- Alya Irdina Binti Ghazali 2020611392 Experiment 2Documento9 páginasAlya Irdina Binti Ghazali 2020611392 Experiment 2ALYA IRDINA BINTI GHAZALI0% (1)
- s4 Chemistry Test Term 2Documento4 páginass4 Chemistry Test Term 2Hulsnator KentAún no hay calificaciones
- UdkdfhvkdhfnfDocumento12 páginasUdkdfhvkdhfnfG M Ali KawsarAún no hay calificaciones
- AQA C2 Past Paper Q&A Part 1Documento184 páginasAQA C2 Past Paper Q&A Part 1Junaid AsgharAún no hay calificaciones
- Chemical EnergeticsDocumento10 páginasChemical EnergeticsShahmeer MahmoodAún no hay calificaciones
- Quiz - 1 - Matter & StoichiometryDocumento4 páginasQuiz - 1 - Matter & StoichiometryDaniel Ngenokesho WandyaAún no hay calificaciones
- British International College: Year 12 Half Term Assessment ChemistryDocumento10 páginasBritish International College: Year 12 Half Term Assessment ChemistryHarry SonAún no hay calificaciones
- British International College: Year 12 Half Term Assessment ChemistryDocumento10 páginasBritish International College: Year 12 Half Term Assessment ChemistryHarry SonAún no hay calificaciones
- Mixed Topic Revision 1 DiffusionDocumento23 páginasMixed Topic Revision 1 DiffusionYaakkwAún no hay calificaciones
- Chem 1 Pre RegeDocumento14 páginasChem 1 Pre RegeFIDEL RONEL OTIENOAún no hay calificaciones
- AS Level Chemistry Bonding ConceptsDocumento24 páginasAS Level Chemistry Bonding Conceptsdanielphilip68Aún no hay calificaciones
- BondingDocumento14 páginasBondingMuizzudin AzaliAún no hay calificaciones
- Ionic Bonding 2 QPDocumento7 páginasIonic Bonding 2 QPHuseyn AgazadeAún no hay calificaciones
- Edexcel Chemistry Unit 6 June 2012 Question PaperDocumento16 páginasEdexcel Chemistry Unit 6 June 2012 Question PaperCharlene ChiaAún no hay calificaciones
- GroupIV WSDocumento14 páginasGroupIV WSMaryam RaiAún no hay calificaciones
- C3Documento12 páginasC3Eunice YeohAún no hay calificaciones
- Compounds Containing The Carbonyl Group QuestionsDocumento69 páginasCompounds Containing The Carbonyl Group QuestionsmarvellousadenugaAún no hay calificaciones
- Edexcel IGCSE May 2012 Chemistry Paper - 2Documento16 páginasEdexcel IGCSE May 2012 Chemistry Paper - 2Coolman PoonAún no hay calificaciones
- Metal p4 Ws 4Documento10 páginasMetal p4 Ws 4NonnoonoAún no hay calificaciones
- Covalent Dative Covalent Bonds 1 QPDocumento10 páginasCovalent Dative Covalent Bonds 1 QPRahbot Wolde-MichaelAún no hay calificaciones
- 4.1 Reactivity of Metals 3 QPDocumento16 páginas4.1 Reactivity of Metals 3 QPDumpsterFireGamingAún no hay calificaciones
- 2.4 2.5 2.6 Assessed HomeworkDocumento7 páginas2.4 2.5 2.6 Assessed HomeworkRabia Rafique100% (1)
- 5th Form Exam ET 2014Documento20 páginas5th Form Exam ET 2014NIRVAN RAMESHAún no hay calificaciones
- SF4 Lewis Structure and Shape of MoleculesDocumento8 páginasSF4 Lewis Structure and Shape of MoleculesSiddhant DuggalAún no hay calificaciones
- The Particulate Nature of Matter 3 QPDocumento10 páginasThe Particulate Nature of Matter 3 QPSajia EhsaniAún no hay calificaciones
- Periodicity Past QuestionsDocumento17 páginasPeriodicity Past QuestionsMohammad KhanAún no hay calificaciones
- "Complex Stuff": Year 13 Unit 5 Test 4 4.4 Transition Metals Answer All Questions Bonne Chance!Documento8 páginas"Complex Stuff": Year 13 Unit 5 Test 4 4.4 Transition Metals Answer All Questions Bonne Chance!Asma AkterAún no hay calificaciones
- Revision ExerciseDocumento7 páginasRevision ExerciseNana BAún no hay calificaciones
- SCLP Samaj School Year 10 Chemistry Revision WorksheetDocumento11 páginasSCLP Samaj School Year 10 Chemistry Revision WorksheetHarshil PatelAún no hay calificaciones
- As-Level Paper 1 Pp12Documento16 páginasAs-Level Paper 1 Pp12faith mAún no hay calificaciones
- Cons. of Mass, Chemical Measurements - Eqns 2 QPDocumento18 páginasCons. of Mass, Chemical Measurements - Eqns 2 QPHusain KamaliAún no hay calificaciones
- Extra moles QuestionsDocumento6 páginasExtra moles QuestionschenzAún no hay calificaciones
- Chemistry 1a N C Principles of Chemistry State of Matter Atoms Atomic StructureDocumento25 páginasChemistry 1a N C Principles of Chemistry State of Matter Atoms Atomic StructuresechosAún no hay calificaciones
- The Particulate Nature of Matter 3 QPDocumento10 páginasThe Particulate Nature of Matter 3 QPBara' HammadehAún no hay calificaciones
- Redox Questions Igcse ChemDocumento7 páginasRedox Questions Igcse ChemCaylinAún no hay calificaciones
- Redox 2 QPDocumento7 páginasRedox 2 QPPramitaAún no hay calificaciones
- Bio Test F5Documento5 páginasBio Test F5Crystal MachipisaAún no hay calificaciones
- Assignment 4 U2 Chem RedoxDocumento34 páginasAssignment 4 U2 Chem RedoxMoshiurAún no hay calificaciones
- Chemical BondingDocumento3 páginasChemical BondingnAún no hay calificaciones
- Gcse Basics 2: © WWW - CHEMSHEETS.co - Uk 09-March-2020 Chemsheets AS 1226 1Documento4 páginasGcse Basics 2: © WWW - CHEMSHEETS.co - Uk 09-March-2020 Chemsheets AS 1226 1Ahmad RazaAún no hay calificaciones
- Chemistry 5070 2023 Gce Question Paper 2Documento8 páginasChemistry 5070 2023 Gce Question Paper 2andrew silungweAún no hay calificaciones
- Igcse Electrochemistry Review PDFDocumento7 páginasIgcse Electrochemistry Review PDFbilly ogadaAún no hay calificaciones
- 2.4, 2.5, 2.6 TestDocumento7 páginas2.4, 2.5, 2.6 Testzafarchem_iqbalAún no hay calificaciones
- 1.3 Question DatabaseDocumento42 páginas1.3 Question DatabasedaminAún no hay calificaciones
- Module 806Documento18 páginasModule 806Hema LataAún no hay calificaciones
- Isomerism 2 QPDocumento9 páginasIsomerism 2 QPPragna AnanthAún no hay calificaciones
- Chapter 10 Exam Style QuestionsDocumento13 páginasChapter 10 Exam Style QuestionsAmanda AnushaAún no hay calificaciones
- Sixth Form 2020 Chemistry ExamDocumento18 páginasSixth Form 2020 Chemistry ExamKitty chenAún no hay calificaciones
- Chapters 22-26 Past Papers QuestionsDocumento10 páginasChapters 22-26 Past Papers QuestionsHehe Haah100% (1)
- Chemical Energetics 2 QPDocumento11 páginasChemical Energetics 2 QPWilliam TsuiAún no hay calificaciones
- CHM1 Structure & Bonding QDocumento115 páginasCHM1 Structure & Bonding QGoutham SivagnanamAún no hay calificaciones
- Ionic Bonding G10Documento6 páginasIonic Bonding G10Mahmoud AladdasiAún no hay calificaciones
- Topic 7 TestDocumento11 páginasTopic 7 Testab9652378Aún no hay calificaciones
- Assignment No 1 BondingDocumento8 páginasAssignment No 1 Bondingmisbah shahidAún no hay calificaciones
- Edexcel Chemistry Topic 3Documento11 páginasEdexcel Chemistry Topic 3locopocpAún no hay calificaciones
- Y12 Unit 1 Test 4 1.4 Periodicity: SECTION A TOTAL ../18 Section B Total /7 TOTAL ./25Documento5 páginasY12 Unit 1 Test 4 1.4 Periodicity: SECTION A TOTAL ../18 Section B Total /7 TOTAL ./25Andrei FloreaAún no hay calificaciones
- 1.4 Assessed HomeworkDocumento5 páginas1.4 Assessed Homeworkchigz09Aún no hay calificaciones
- AtomicStructure HomeworkDocumento6 páginasAtomicStructure HomeworkAndrei FloreaAún no hay calificaciones
- Atomic Structure Questions Relative Atomic Mass, Relative Molecular Mass & Mass SpectrosDocumento2 páginasAtomic Structure Questions Relative Atomic Mass, Relative Molecular Mass & Mass SpectrosAndrei FloreaAún no hay calificaciones
- Bonding RevisionDocumento7 páginasBonding RevisionAndrei FloreaAún no hay calificaciones
- Romanian Coat of Arms Arge General Ș School Inspectorate LogoDocumento1 páginaRomanian Coat of Arms Arge General Ș School Inspectorate LogoAndrei FloreaAún no hay calificaciones
- Atomic Structure Questions Relative Atomic Mass, Relative Molecular Mass & Mass SpectrosDocumento2 páginasAtomic Structure Questions Relative Atomic Mass, Relative Molecular Mass & Mass SpectrosAndrei FloreaAún no hay calificaciones
- Program VaraDocumento1 páginaProgram VaraAndrei FloreaAún no hay calificaciones
- Salt HydrolysisDocumento3 páginasSalt Hydrolysisna_napanda100% (1)
- Composition of Grey Cast IronDocumento2 páginasComposition of Grey Cast IronPranil GhatageAún no hay calificaciones
- Lesson Plan in ScienceDocumento4 páginasLesson Plan in ScienceDaryl HilongoAún no hay calificaciones
- Astm A572-50Documento1 páginaAstm A572-50anumnedAún no hay calificaciones
- Question Bank On Electronic ConfigurationDocumento4 páginasQuestion Bank On Electronic ConfigurationRaju SinghAún no hay calificaciones
- Gasenje Pozara Solarnih Panela 2019Documento23 páginasGasenje Pozara Solarnih Panela 2019Alen ŠkudarAún no hay calificaciones
- Ch4 Watertreatment DisinfectionDocumento30 páginasCh4 Watertreatment DisinfectionSUBHAM KumarAún no hay calificaciones
- Phosphorus - WikipediaDocumento25 páginasPhosphorus - Wikipediatsvmpm1765Aún no hay calificaciones
- 5 Whmis SymbolsDocumento3 páginas5 Whmis Symbolsapi-369690183Aún no hay calificaciones
- Co-Ordination Compound Ex-4 Solution For Vedantu TatvaDocumento9 páginasCo-Ordination Compound Ex-4 Solution For Vedantu TatvaAbhinav ThapliyalAún no hay calificaciones
- Electron Configuration and Periodicity - Paper 2Documento30 páginasElectron Configuration and Periodicity - Paper 2elenaAún no hay calificaciones
- 2013 MJC h2 Chem Prelim p2 QPDocumento28 páginas2013 MJC h2 Chem Prelim p2 QPSiaw MinAún no hay calificaciones
- Titration CalculationsDocumento11 páginasTitration CalculationsYuones BalahAún no hay calificaciones
- Emission Regulations Part - 2 PDFDocumento24 páginasEmission Regulations Part - 2 PDFAkul SenapatiAún no hay calificaciones
- FMTS-C PsDocumento2 páginasFMTS-C PspostscriptAún no hay calificaciones
- Periodic Classification of Elements-10282920199034123Documento22 páginasPeriodic Classification of Elements-10282920199034123Hitesh GargAún no hay calificaciones
- Asme Sec Viii D1 C PT Ucl PDFDocumento7 páginasAsme Sec Viii D1 C PT Ucl PDFsuraj kumarAún no hay calificaciones
- International Material GradeDocumento7 páginasInternational Material GradeDvs RameshAún no hay calificaciones
- Welding ElectrodesDocumento19 páginasWelding Electrodesraju100% (1)
- FUNCHEM.2 2021 Slides 2021 2Documento27 páginasFUNCHEM.2 2021 Slides 2021 2shabanaAún no hay calificaciones
- Disc Springs ScreenDocumento20 páginasDisc Springs ScreenharripottsAún no hay calificaciones
- 10eng PDFDocumento18 páginas10eng PDFАхмед АбдуллаAún no hay calificaciones
- AssessmentDocumento8 páginasAssessmentdilsharakavi100% (1)
- 0653 w16 QP 32Documento20 páginas0653 w16 QP 32yuke kristinaAún no hay calificaciones
- Chemistry 0620 - 2011 - QPDocumento12 páginasChemistry 0620 - 2011 - QPMinakshiAún no hay calificaciones
- Al WeldingDocumento70 páginasAl WeldingTuyen NguyenAún no hay calificaciones
- Halogen Derivatives-1Documento9 páginasHalogen Derivatives-1avishkarshinde00Aún no hay calificaciones