Documentos de Académico
Documentos de Profesional
Documentos de Cultura
Exp 2 Bubble Cap Distillation
Cargado por
Faris HamirDerechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Exp 2 Bubble Cap Distillation
Cargado por
Faris HamirCopyright:
Formatos disponibles
Mass Transfer I
CLB 20803
Experiment 2
BUBBLE CAP DISTILLATION PROCESS
1.0
OBJECTIVES
2.0
To operate vapor liquid separation process using a Bubble Cap Distillation
Unit.
To analyze the sample for the Top and Bottom Product by Refractometer to
obtain the Refractive Index in order to determine their respective composition.
To calculate the number of stages by using McCabe Thiele method
OVERVIEW
Distillation is a separation method in which mixture components in a liquid mixture are
separated based on their relative volatilities. The distillation column provides an environment
where the liquid and vapour phases can approach equilibrium within a column. In the packed
column there is high surface area for contact between the vapor and liquid, whereas the plate
column provides distinct stages at which equilibrium can be approached. Separation is achieved
by condensed vapor flowing as a liquid down the column theoretically achieving equilibrium with
the vapor flowing up the column. The distribution of components differs in each phase and results
in the separation. In the case of a binary mixture in batch distillation under total reflux, the vapor
condensing at the top of the distillation column will be rich in one of the components. Liquid
leaving the reboiler at the base of the column rich in the other component. As the system reaches
equilibrium the separation process reaches steady state for that apparatus and set of operating
conditions. This experiment is designed to study the distillation of a binary mixture of Ethanol
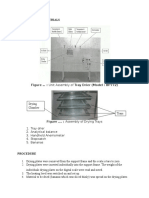
Water in a tray distillation column. Figure 1 is a sketch of a tray with bubble cap and Figure 2
shows the schematic diagram of the apparatus in this experiment.
Figure 1: Bubble cap tray
FG/exp2/July2012
Mass Transfer I
CLB 20803
Figure 2: Bubble Cap distillation column
3.0
Experimental Procedures
3.1
Chemicals and ancillary equipments required:
Chemicals Required.
Ethanol industrial grade.
Deionized-Water
Ancillary Equipments Required:
Two 2000ml Jug and two 25ml beakers.
Refractometer for measurement of sample concentrations.
Calibration Curve of ethanol-water mixtures.
3.2 Calibration curve of ethanol-water mixtures
1. Prepare a 5% of ethanol-water solution in a test tube.
2. Using a refractometer, obtain the refractive index reading for solution.
FG/exp2/July2012
Mass Transfer I
CLB 20803
3. Using increments of 5%, obtain refractive index readings for ethanol-water mixtures
up to 100%.
4. Plot a refractive index vs % of concentration graph.
3.3
General start-up procedures.
1. Prepare 30 litres of 10 % mixture of ethanol in water by mixing appropriate
quantities of the Industrial Grade of ethanol in distilled Water.
2. Ensure valves V1, RCV1, V3, V4, V5, V6, V7, V9 and RCV2 are closed.
3. Ensure valves V2, V8 and V10 are open.
4. Ensure that the Bottom Product sampling valve V5 and Top Product sampling valve
V4 is closed.
5. Slowly turn on the Cooling Water to the Condenser. Make sure water is indeed
flowing through it.
6. Check water flows to the drain from the Cooling Water outlets. Do not start the
experiment until the Cooling Water flow is visible in the outlets to the drain.
7. Set water pressure to 6 L/min.
8. Ensure that the Reboiler Vessel is already charged with the Ethanol Water mixture
through the Charge Port (Refer to the diagram).Fill the reboiler vessel with the
Ethanol-Water mixtures.
9. Check that the liquid level in Reboiler vessel is satisfactory (Refer to instructor /
technician).Otherwise top up with the Ethanol Water mixture through the Charge
Port (Refer to instructor / technician).
10. Ensure that the concentration of feed in the reboiler is correct and the liquid level is
at the vessel equator. (Refer to Lecturer / Technician).
11. The Reboiler Vessel should never be filled with liquid above the maximum level
indicated. The level should never recede below the minimum level while on
operation.
12. Obtain a sample of the ethanol-water mixture from the reboiler through valve V7 and
test its composition with the refractometer.
13. Turn on the main power control switch.
14. Turn on the power switch of the heater.
15. Switch on electrical supply (green heater on button).
16. Set heater controller HC4 to maximum setting (about 230V@ 30A).
17. Close the Reflux Adjustment Valve and valve below Product Cooler.
18. When distillate liquid is seen to flow through R1.A1, reduce the heat input and set
HC.4 to roughly 20A.
19. Allow a period of 15 minutes for the equipment to maintain thermal equilibrium with
surroundings.
20. The unit is now ready to be used for an experiment.
21. Switch on the heaters of the reboiler unit at the control panel and wait until the
mixture is seen boiling and vapor is seen to reach the top of the column
3.4
Experiment: Operation under reflux ratio condition
1. Ensure that the valves for reflux ratio RCV adjustment are opened.
2. Adjust both valve for Reflux Ratio RCV1 and valve below product cooler V2 so that
both readings on R1 and R2 provide a reflux ratio of 1.0 for the operation.
FG/exp2/July2012
Mass Transfer I
CLB 20803
3. Wait for about 15 minutes until the flow rate shown by R1 and the temperature
readings at the top and bottom of the columns are steady. (Refer to instructor /
technician).
4. Collect samples from the Reboiler and the overhead from the Sampling Port for every
5 minutes.
5. Observe the temperature of the reboiler T14.If the temperature is already 90 OC,
reduce the current of the reboiler to between 10A to 15A.
6. Observe the flow rate at R1. Adjust the valve for Reflux Ratio adjustment to ensure
that the Reflux Ratio is maintained at 1.0.
7. The concentration of the samples drawn is measure using the refractive index method
8. Record the reading of temperature for every 5 minutes
9. Readings are recorded in the provided table of results.
10. Then, the experiment is repeated with Reflux ration of 1.5 and 2.0.
3.5
General shut down procedures.
1.
2.
3.
4.
5.
Adjust heater controller HC.4 to minimum setting.
Switch off electrical supply (red heater off button).
Turn off the power switch of the reboiler.
Turn off the Main power control switch.
Do not drain the hot liquid from the Reboiler. If necessary, the liquid within the
system could be drained only when the liquid is already cooled.
6. Allow the cooling water to run for some time.
4.0
ANALYSIS AND DISCUSSION
Discuss all your results. The questions below only serve as a guideline. Your discussion
should not only limit to these questions.
1. Use the table for data collection in Appendix B.
2. Plot Calibration Curve for ethanol-water mixtures (Refractive index vs %
concentration.
3. Referring to calibration curve, estimate the mole fraction ethanol of the Top and
Bottom product samples for each reflux.
4. Plot the Equilibrium Curve for ethanol-water mixture. The data is given in Appendix
A.
5. Calculate the complete overall and component mass balance of the process.
6. By using McCabe Thiele Method, construct the graph to determine the number of
stages that occur in the processes for each reflux.
7. Discuss the results obtained and effect of reflux ratio for ethanol-water mixture
separation
8. Discuss any possible errors in the experiment and state any recommendation to
improve the process.
5.0
REFERENCES
1. Treybal, R.E., Mass Transfer Operations, 3rd ed., Mc-Graw-Hill, 1981
2. McCabe & Smith, Unit Operations of Chemical Engineering, 5th ed, Mc-Graw-Hill,
1993
3. Geankoplis,C. J. Z., Mass Transport Phenomena, 4th Ed., Rine Hart Winston, New
York.
FG/exp2/July2012
Mass Transfer I
CLB 20803
4. Coulson & Richardson, Chemical Engineering. Vol. 2 Pergamon Press, Oxford.
APPENDIX A
Equilibrium Data for Ethanol-Water Mixtures
Mole fraction of ethanol in liquid, x
0.00
0.05
0.10
0.40
0.60
0.80
0.94
0.90
0.94
0.96
0.98
Mole fraction of ethanol in vapor, y
0.00
0.38
0.53
0.75
0.79
0.86
0.94
0.91
0.94
0.96
0.99
FG/exp2/July2012
Mass Transfer I
CLB 20803
APPENDIX B
Table of Results
Reflux ratio
= 1.0
Rotameter reading R1 (L/hr)
= __________________
Rotameter reading R2 (L/hr)
= __________________
Temperature T4 (oC)
= __________________
Temperature T2 (oC)
= __________________
Time, t
(min)
TOP PRODUCT
Refractive index
Mole fraction
(RI)
BOTTOM PRODUCT
Refractive index
Mole fraction
(RI)
0
5
10
15
20
25
30
Reflux ratio
= 1.5
Rotameter reading R1 (L/hr)
= __________________
Rotameter reading R2 (L/hr)
= __________________
Temperature T4 (oC)
= __________________
Temperature T2 (oC)
= __________________
Time, t
(min)
TOP PRODUCT
Refractive index
Mole fraction
(RI)
BOTTOM PRODUCT
Refractive index
Mole fraction
(RI)
0
5
10
15
FG/exp2/July2012
Mass Transfer I
CLB 20803
20
25
30
Reflux ratio
= 2.0
Rotameter reading R1 (L/hr)
= __________________
Rotameter reading R2 (L/hr)
= __________________
Temperature T4 (oC)
= __________________
Temperature T2 (oC)
= __________________
Time, t
(min)
TOP PRODUCT
Refractive index
Mole fraction
(RI)
BOTTOM PRODUCT
Refractive index
Mole fraction
(RI)
0
5
10
15
20
25
30
FG/exp2/July2012
También podría gustarte
- Exp - 2 Bubble Cap Distillation ColumnDocumento13 páginasExp - 2 Bubble Cap Distillation ColumnAdawiyah Al-jufri100% (1)
- Exp 06 - Distillation ColumnDocumento11 páginasExp 06 - Distillation ColumnAli AhmadAún no hay calificaciones
- Bubble Cap Distillation ColumnDocumento3 páginasBubble Cap Distillation Columnnhalieza1067Aún no hay calificaciones
- CPB 20104 Mass Transfer 2 UniKL MICET Experiment 3: Plate and Frame Filter Press Full Lab ReportDocumento13 páginasCPB 20104 Mass Transfer 2 UniKL MICET Experiment 3: Plate and Frame Filter Press Full Lab ReportSiti Hajar Mohamed100% (4)
- CKB 20104 Reaction Engineering UniKL MICET Experiment 1a: The Batch Saponification of Ethyl Acetate Full Lab ReportDocumento11 páginasCKB 20104 Reaction Engineering UniKL MICET Experiment 1a: The Batch Saponification of Ethyl Acetate Full Lab ReportSiti Hajar Mohamed82% (11)
- Lab Report CSTR RTDDocumento13 páginasLab Report CSTR RTDNurul IzzahAún no hay calificaciones
- Isothermal Batch ReactorDocumento10 páginasIsothermal Batch ReactorSaswiny Ritchie0% (2)
- Experiment: Packed Distillation ColumnDocumento4 páginasExperiment: Packed Distillation Columnnhalieza1067Aún no hay calificaciones
- Vapor Liquid EquilibriumDocumento7 páginasVapor Liquid Equilibriummahbub1332100% (1)
- Apparatus, Procedure, Recommendation Tray DryerDocumento4 páginasApparatus, Procedure, Recommendation Tray DryerillyzlAún no hay calificaciones
- Plug Flow Reactor Experiment ReportDocumento25 páginasPlug Flow Reactor Experiment ReportCesarah Cabungcal100% (1)
- Lab 10-Batch ReactorDocumento22 páginasLab 10-Batch Reactorniraj_bairagiAún no hay calificaciones
- Batch Distillation Laboratory ReportDocumento17 páginasBatch Distillation Laboratory ReportNayantara Soni100% (1)
- Calibration of Peristaltic Pumps - Lab 1Documento12 páginasCalibration of Peristaltic Pumps - Lab 1mmccomas08100% (2)
- Problem Set # 2Documento5 páginasProblem Set # 2Dharyl Flores0% (2)
- Understanding Reaction Kinetics in Batch and Continuous ReactorsDocumento14 páginasUnderstanding Reaction Kinetics in Batch and Continuous ReactorsAmy Farhana33% (3)
- Absorption of Carbon Dioxide Into WaterDocumento11 páginasAbsorption of Carbon Dioxide Into WaterEstelle Jean CauilanAún no hay calificaciones
- Rate of Saponification Reaction in Batch ReactorDocumento21 páginasRate of Saponification Reaction in Batch ReactorLaila Al-shafieAún no hay calificaciones
- Climbing FilmDocumento34 páginasClimbing FilmTunji Aminu100% (1)
- Oil Distillation ReportDocumento10 páginasOil Distillation ReportnisasoberiAún no hay calificaciones
- Batch Distillation - Lab ReportDocumento21 páginasBatch Distillation - Lab ReportAngelica Joyce Benito100% (1)
- CSTR 40L LAB EXPERIMENTDocumento18 páginasCSTR 40L LAB EXPERIMENTSaber Minato Azrul100% (2)
- Vle UnitDocumento26 páginasVle UnitAhmad Ifwat50% (2)
- Lab Report 5Documento12 páginasLab Report 5Norhanisah Zamri Rcsu100% (1)
- Lab10 CompleteDocumento22 páginasLab10 CompleteMastura Ahmad Termizi100% (1)
- Lab Report TPP Experiment 3Documento10 páginasLab Report TPP Experiment 3Nurul Najwa100% (1)
- Climbing Film EvaporatorDocumento8 páginasClimbing Film Evaporatorsaz140% (1)
- VLE Unit (Complete)Documento26 páginasVLE Unit (Complete)hishamAún no hay calificaciones
- Packed Bed Distillation Column Lab ReportDocumento13 páginasPacked Bed Distillation Column Lab ReportShamini Sathivel100% (6)
- Batch Sedimentation Post-Laboratory Experiment 4Documento8 páginasBatch Sedimentation Post-Laboratory Experiment 4Kat-kat GrandeAún no hay calificaciones
- Distillation Column Pressure Drop & Refractive IndexDocumento18 páginasDistillation Column Pressure Drop & Refractive IndexAmir Al-AimanAún no hay calificaciones
- Exp - S1A - Solid in Air DiffusionDocumento7 páginasExp - S1A - Solid in Air DiffusionAnuj SrivastavaAún no hay calificaciones
- Vapor-Liquid Equilibria of CCl4-Toluene MixtureDocumento5 páginasVapor-Liquid Equilibria of CCl4-Toluene MixtureAakash Sharma100% (1)
- Isothermal Semi-Batch Reactor PPT RJC SirDocumento16 páginasIsothermal Semi-Batch Reactor PPT RJC Sirsdjdsf100% (1)
- Tray DryerDocumento16 páginasTray Dryermirdza94Aún no hay calificaciones
- Liquid Liquid ExtractionDocumento16 páginasLiquid Liquid ExtractionShahrizatSmailKassimAún no hay calificaciones
- Gas Absorption: Determining Drag and Flooding FlowsDocumento5 páginasGas Absorption: Determining Drag and Flooding FlowsDean Joyce AlborotoAún no hay calificaciones
- Experiment 1B - Tubular ReactorDocumento14 páginasExperiment 1B - Tubular ReactorNajmul Puda PappadamAún no hay calificaciones
- LabReport Gas DiffusionDocumento21 páginasLabReport Gas DiffusionSharing Caring83% (6)
- Energy BalanceDocumento16 páginasEnergy BalancewizlanAún no hay calificaciones
- Activation Energy of an Ionic ReactionDocumento10 páginasActivation Energy of an Ionic ReactionHanif YusofAún no hay calificaciones
- Lab Report CSTR 1Documento16 páginasLab Report CSTR 1Nisha SharifAún no hay calificaciones
- Hydrodynamics of Packed ColomnDocumento6 páginasHydrodynamics of Packed ColomnDhananjay KadamAún no hay calificaciones
- Sieve Plate Distillation Column - Lab ReportDocumento4 páginasSieve Plate Distillation Column - Lab ReportShrankhla NaryaAún no hay calificaciones
- Experiment 3 Pulsed Column Liquid-Liquid Extraction ColumnDocumento13 páginasExperiment 3 Pulsed Column Liquid-Liquid Extraction ColumnFikri IlyasAún no hay calificaciones
- Absorption in Packed Bed Lab ManualDocumento5 páginasAbsorption in Packed Bed Lab ManualAshish Verma100% (1)
- ELA Heat of SolutionDocumento15 páginasELA Heat of SolutionJimAún no hay calificaciones
- Optimized Solids Suspension: Achieving Uniform Dispersion is Critical to Product QualityDocumento7 páginasOptimized Solids Suspension: Achieving Uniform Dispersion is Critical to Product QualitymichsantosAún no hay calificaciones
- Intro CSTRDocumento6 páginasIntro CSTREmmanuel PlazaAún no hay calificaciones
- Exp 4 Batch Evaporative Crystallization PDFDocumento9 páginasExp 4 Batch Evaporative Crystallization PDFmirza farhanAún no hay calificaciones
- CKB 20104 Reaction Engineering UniKL MICET Experiment 2a Effect of RTD On The Reaction in CSTR Full Lab ReportDocumento29 páginasCKB 20104 Reaction Engineering UniKL MICET Experiment 2a Effect of RTD On The Reaction in CSTR Full Lab ReportSiti Hajar Mohamed100% (6)
- Fluidisation ReportDocumento29 páginasFluidisation ReportBenjamin Jie100% (2)
- Exp 3-Vapor-Liquid Equilibrium UnitDocumento18 páginasExp 3-Vapor-Liquid Equilibrium UnitKhairulAzwanizam100% (2)
- Sieve Plate Distillation ColumnDocumento9 páginasSieve Plate Distillation ColumnAshish VermaAún no hay calificaciones
- Exp 1 Packed Column DistillationDocumento12 páginasExp 1 Packed Column DistillationLuqman WasirAún no hay calificaciones
- Continuous Distillation Lab ExperimentDocumento3 páginasContinuous Distillation Lab ExperimentHusna Hafiza Bt. R.AzamiAún no hay calificaciones
- Report Distillation ColumnDocumento20 páginasReport Distillation ColumnAzam Najmi33% (3)
- Experiment 2 - Study of Packed Column DistillationDocumento7 páginasExperiment 2 - Study of Packed Column DistillationAdawiyah Az-zahra100% (1)
- Batch Reactive DistillationDocumento7 páginasBatch Reactive DistillationChalmer BelaroAún no hay calificaciones
- Process Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersDe EverandProcess Steam Systems: A Practical Guide for Operators, Maintainers, and DesignersAún no hay calificaciones
- FatDocumento7 páginasFatFaris HamirAún no hay calificaciones
- Tutorial 3Documento1 páginaTutorial 3Faris HamirAún no hay calificaciones
- Distillate MechDocumento3 páginasDistillate MechFaris HamirAún no hay calificaciones
- Tutorial 1 - Introduction To Analytical and Organic ChemistryDocumento2 páginasTutorial 1 - Introduction To Analytical and Organic ChemistryFaris HamirAún no hay calificaciones
- Experiment 4-Oil Water SeparationDocumento10 páginasExperiment 4-Oil Water SeparationFaris Hamir67% (3)
- Hydrogen Design 2520of 2520equipmentsDocumento37 páginasHydrogen Design 2520of 2520equipmentsjavilapiedraAún no hay calificaciones
- Jar TestingDocumento11 páginasJar TestingFaris HamirAún no hay calificaciones
- PDocumento1 páginaPFaris HamirAún no hay calificaciones
- Lab Manual Jan2013 Air FlowDocumento5 páginasLab Manual Jan2013 Air FlowLaila FaeizahAún no hay calificaciones
- 1 s2.0 S0304389409019104 MainDocumento9 páginas1 s2.0 S0304389409019104 MainFaris HamirAún no hay calificaciones
- 1 s2.0 S1385894711010163 MainDocumento9 páginas1 s2.0 S1385894711010163 MainFaris HamirAún no hay calificaciones
- Redesign Jobs Improve Morale Bank EmployeesDocumento2 páginasRedesign Jobs Improve Morale Bank EmployeesFaris HamirAún no hay calificaciones
- Teambuilding Game. (Faris N Apau)Documento4 páginasTeambuilding Game. (Faris N Apau)Faris HamirAún no hay calificaciones
- PDocumento1 páginaPFaris HamirAún no hay calificaciones
- Tutorial 1 - HumidificationDocumento2 páginasTutorial 1 - HumidificationwafinAún no hay calificaciones
- Tutorial Exp 4Documento4 páginasTutorial Exp 4Faris Hamir100% (4)
- Lab Manual Jan2013 Air FlowDocumento5 páginasLab Manual Jan2013 Air FlowLaila FaeizahAún no hay calificaciones
- FontsDocumento1 páginaFontsFaris HamirAún no hay calificaciones
- Global WarmDocumento13 páginasGlobal WarmFaris HamirAún no hay calificaciones
- Obj. Activities 2010 2011 2012 2013 Sem 1 Sem 2 Sem 3 Sem 4 Sem 5 Sem 6Documento1 páginaObj. Activities 2010 2011 2012 2013 Sem 1 Sem 2 Sem 3 Sem 4 Sem 5 Sem 6Faris HamirAún no hay calificaciones
- Nitric AcidDocumento4 páginasNitric Acidmuneeb907Aún no hay calificaciones
- The Realization of Global WarmingDocumento5 páginasThe Realization of Global WarmingFaris HamirAún no hay calificaciones
- Impact of Improper Waste Management On EnvironmentDocumento2 páginasImpact of Improper Waste Management On EnvironmentKuan EeAún no hay calificaciones
- Propulsion of Sea ShipDocumento15 páginasPropulsion of Sea ShipFaris Hamir100% (1)
- ErtDocumento2 páginasErtFaris HamirAún no hay calificaciones
- ErtDocumento2 páginasErtFaris HamirAún no hay calificaciones
- Global WarmingDocumento1 páginaGlobal WarmingvlatkomkAún no hay calificaciones
- The Realization of Global WarmingDocumento5 páginasThe Realization of Global WarmingFaris HamirAún no hay calificaciones
- Exp 4 Centrifugal CompressorDocumento11 páginasExp 4 Centrifugal CompressorFaris HamirAún no hay calificaciones
- 12 New Trends in ManagementDocumento18 páginas12 New Trends in ManagementSaqib IqbalAún no hay calificaciones
- Simulation of BJT Amplifier: Course - Section: ECE20L-E06 Group NumberDocumento10 páginasSimulation of BJT Amplifier: Course - Section: ECE20L-E06 Group NumberLuch ÜAún no hay calificaciones
- Despiece Des40330 Fagor Sr-23Documento45 páginasDespiece Des40330 Fagor Sr-23Nữa Đi EmAún no hay calificaciones
- Texts Hugues de VarineDocumento15 páginasTexts Hugues de VarineInteractionsonlineAún no hay calificaciones
- 7 Barriers To Implementing and Maintaining An Effective HRM FunctionDocumento13 páginas7 Barriers To Implementing and Maintaining An Effective HRM FunctionPaing Hein KyawAún no hay calificaciones
- Steam Circulation SystemDocumento36 páginasSteam Circulation Systemjkhan_724384100% (1)
- Latka March2020 DigitalDocumento68 páginasLatka March2020 DigitalDan100% (2)
- A Thesis 123Documento77 páginasA Thesis 123Meli SafiraAún no hay calificaciones
- 4MA1 1H Que 20210304Documento28 páginas4MA1 1H Que 20210304mo gaAún no hay calificaciones
- Manifest Merger Debug ReportDocumento14 páginasManifest Merger Debug ReportRam PankhaniyaAún no hay calificaciones
- Thrust Equation For A Turbofan Double Inlet/Outlet: Joshtheengineer April 8, 2017Documento7 páginasThrust Equation For A Turbofan Double Inlet/Outlet: Joshtheengineer April 8, 2017Muhammad RidwanAún no hay calificaciones
- 660 Inventions That Changed Our WorldDocumento5 páginas660 Inventions That Changed Our WorldKoby RamosAún no hay calificaciones
- What Is A BibliographyDocumento7 páginasWhat Is A BibliographyKaye Diamante ValleserAún no hay calificaciones
- Name: Keatlaretse Bridgette Surname: Macucwa Module Name: Social Work Practice Module Code: BSW 2605 Assessment No: 2 Due Date: 14 August 2020Documento6 páginasName: Keatlaretse Bridgette Surname: Macucwa Module Name: Social Work Practice Module Code: BSW 2605 Assessment No: 2 Due Date: 14 August 2020keatlaretse macucwaAún no hay calificaciones
- Stakeholder RegisterDocumento7 páginasStakeholder Registerrouzbehk6515Aún no hay calificaciones
- Risk Culture Assessment QuestionnaireDocumento3 páginasRisk Culture Assessment QuestionnairemohamedAún no hay calificaciones
- CE ENG HL660,660L, HL665,665L AUG2018 Rev.0 WebDocumento4 páginasCE ENG HL660,660L, HL665,665L AUG2018 Rev.0 WebJohn LeonneAún no hay calificaciones
- 2016 Book IrrigationAndDrainageEngineeriDocumento747 páginas2016 Book IrrigationAndDrainageEngineeriJesús Garre Ruiz100% (2)
- Piper Lance II - Turbo Lance II-Maintenance - smv1986Documento568 páginasPiper Lance II - Turbo Lance II-Maintenance - smv1986willkobiAún no hay calificaciones
- Economics and Its NatureDocumento4 páginasEconomics and Its NatureElrey IncisoAún no hay calificaciones
- Katie Nelson PDFDocumento3 páginasKatie Nelson PDFKatie NAún no hay calificaciones
- Rip Bushing PDFDocumento38 páginasRip Bushing PDFTravis Wood100% (1)
- Digital Bending Machine User Manual - BD SeriesDocumento7 páginasDigital Bending Machine User Manual - BD SeriesTatiane Silva BarbosaAún no hay calificaciones
- 7 ReferencesDocumento6 páginas7 Referencessuneerav17100% (1)
- ARHITECTURA Si FOCULDocumento282 páginasARHITECTURA Si FOCULTheodor DinuAún no hay calificaciones
- 574-Article Text-1139-1-10-20170930Documento12 páginas574-Article Text-1139-1-10-20170930Jhufry GhanterAún no hay calificaciones
- Critical Regionalism in ArchitectureDocumento75 páginasCritical Regionalism in ArchitecturebranishAún no hay calificaciones
- Speech Language Impairment - Eduu 511Documento15 páginasSpeech Language Impairment - Eduu 511api-549169454Aún no hay calificaciones
- MFS 7104 Quantitative TechniquesDocumento2 páginasMFS 7104 Quantitative TechniquesDavid KAún no hay calificaciones
- ChuzaChen Hydroelectric Power ProjectDocumento13 páginasChuzaChen Hydroelectric Power ProjectkanabaramitAún no hay calificaciones