Documentos de Académico
Documentos de Profesional
Documentos de Cultura
From Van Der Waals To VVPR, JSF, 55,2010,438-447
Cargado por
Gustavo Adolfo RodriguezDescripción original:
Título original
Derechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
From Van Der Waals To VVPR, JSF, 55,2010,438-447
Cargado por
Gustavo Adolfo RodriguezCopyright:
Formatos disponibles
J.
of Supercritical Fluids 55 (2010) 438447
Contents lists available at ScienceDirect
The Journal of Supercritical Fluids
j our nal homepage: www. el sevi er . com/ l ocat e/ supf l u
From van der Waals to VTPR: The systematic improvement of the van der Waals
equation of state
Bastian Schmid, Jrgen Gmehling
Carl von Ossietzky Universitt Oldenburg, Technische Chemie, D-26111 Oldenburg, Germany
a r t i c l e i n f o
Article history:
Received 30 August 2010
Received in revised form 13 October 2010
Accepted 13 October 2010
Keywords:
van der Waals equation of state
VTPR
Group contribution equation of state
Process design
a b s t r a c t
Today the synthesis, design and optimization of the different processes in the oil and gas processing,
pharmaceutical, food and environmental industry is carried out by solving the balance equations of
a mathematical model of the unit operation or the whole plant with the help of process simulators.
The accuracy of the simulation mainly depends on the quality of the thermophysical properties used.
Besides kinetic data for the chemical reaction, heat and mass transfer, pure component properties and in
particular a reliable knowledge of the phase equilibrium behavior of the system is required. Therefore,
the development of powerful thermodynamic models was a very important step in the right direction.
For the description of the required distribution coefcients or separation factors, reliable thermodynamic
models are required. For a long time, the application of g
E
-models as well as group contribution methods
was most popular. However, equations of state showa lot of advantages compared to g
E
-models. Besides
the phase equilibrium of subcritical compounds, they can handle supercritical compounds. At the same
time other important properties such as densities, heat capacities, and enthalpies of the pure compounds
and their mixtures are obtained. Starting fromthe cubic van der Waals equation of state, after important
improvements today the volume translated PengRobinson group contribution equation can be applied
successfully for process development and design.
2010 Elsevier B.V. All rights reserved.
1. Introduction
Starting from the isofugacity condition, two different
approaches can be applied. For vaporliquid equilibria, one
can start from the following approaches:
A : x
i
i
f
o
i
= y
i
V
i
P (1.1)
B : x
i
L
i
= y
i
V
i
(1.2)
Approach A, also known as approach, is used for g
E
- or group
contribution models, such as UNIFAC [1] or modied UNIFAC [2].
The reality of the liquid phase is taken into account using activity
coefcients. As standardfugacity f
o
i
, usually the fugacity of the pure
liquid at system temperature and pressure is chosen, which can be
calculated following Eq. (1.3)
f
o
i
(T, P
s
i
) =
L
i
P
s
i
=
V
i
P
s
i
s
i
P
s
i
(1.3)
where the fugacity coefcients
L
i
resp.
V
i
are replaced by the
fugacitycoefcient
s
i
inthesaturationstate. At systempressurethe
Corresponding author. Tel.: +49 441 798-3831; fax: +49 441 798 3330.
E-mail address: gmehling@tech.chem.uni-oldenburg.de (J. Gmehling).
URL: http://www.uni-oldenburg.de/tchemie (J. Gmehling).
inuence of the pressure difference P P
s
i
on the standard fugacity
can be taken into account with the help of the Poynting factor Poy
i
.
f
o
i
(T, P) =
s
i
P
s
i
Poy
i
(1.4)
The combination of Eqs. (1.1) and (1.4) lead to
x
i
s
i
P
s
i
Poy
i
= y
i
V
i
P (1.5)
Inthecaseof moderatepressures andonlyslightlyassociatingcom-
pounds, the following approximation can be made:
s
i
Poy
i
V
i
(1.6)
which lead to a simplied relation (Eq. (1.7)).
x
i
i
P
s
i
y
i
P (1.7)
The approach B, uses fugacity coefcients to account for the
real behavior in both the liquid and the vapor phase. For the cal-
culation an equation of state with reliable mixing rules has to be
used to describe the pressurevolumetemperature behavior as a
function of composition of the liquid and the vapor phase.
In1873Johannes Diderikvander Waals presentedthe rst cubic
equation of state in his doctoral thesis. With his cubic equation of
state for the rst time effects like evaporation and condensation,
the two phase region and critical phenomena could be described
with only two parameters a and b. The application of equations of
0896-8446/$ see front matter 2010 Elsevier B.V. All rights reserved.
doi:10.1016/j.supu.2010.10.018
B. Schmid, J. Gmehling / J. of Supercritical Fluids 55 (2010) 438447 439
state has a lot of advantages compared to g
E
-models. Besides the
possibility to describe supercritical systems, at the same time other
thermophysical properties for process design, such as densities,
enthalpies, and molar heat capacities, can be obtained.
The systematic improvements of the cubic van der Waals equa-
tion of state led nally to VTPR, the universal volume translated
PengRobinson group contribution equation of state.
2. From the van der Waals equation of state to the group
contribution equation of state VTPR
The cubic van der Waals equation of state (Eq. (2.1)) consists of
a repulsive and an attractive part [3].
P =
R T
v b
a
v
2
(2.1)
The parameter a takes into account the attractive forces between
the different molecules andparameter b describes the closest pack-
ing volume. Both parameters can directly be obtained from critical
data, since at the critical point the critical isotherm shows a saddle
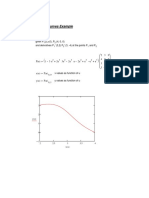
point. This can be seen from Fig. 1, which shows the Pv behavior
of a pure component.
The disadvantages of the van der Waals equation of state are the
very poor results for the liquid densities and the vapor pressures.
This can be seen fromFig. 2. In Fig. 2 the calculated liquid and vapor
densities of methanol in the saturation state are shown together
with the densities obtained by other equations of state together
with the experimental data [4] for a reduced temperature range
from 0.5 to 1.
To improve the results, a large number of modied equations
of state were published. An important improvement was achieved
by the equation of state published by Redlich and Kwong [5]. They
modied the attractive term and introduced the rst temperature
dependency of the attractive term.
2.1. The RedlichKwong equation of state
P =
R T
v b
a
T
0.5
v(v +b)
(2.2)
The RedlichKwong equation delivers improved results for vapor
pressures but still poor results for liquid densities.
Fig. 1. Pv-diagram of a pure compound.
Fig. 2. Experimental () and predicted densities for methanol inthe saturationstate
usingVTPR(), PengRobinson( ), RedlichKwong() andvander Waals (
. . .
).
2.2. The SoaveRedlichKwong equation of state
The introduction of a temperature dependent attractive param-
eter a(T) realized in the SoaveRedlichKwong equation of state
(Eq. (2.3)) was an important step in the right direction [6].
P =
R T
v b
a(T)
v(v +b)
(2.3)
Great improvement in the description of the vapor pressures
were obtained by Soave [6] who introduced a temperature depen-
dency of the attractive parameter a with the help of a -function,
where the acentric factor is used as additional parameter. One
can distinguish between generalized -functions Eq. (2.4) and -
functions such as the MathiasCopeman or Twu -function, where
the required parameters are tted directly to experimental pure
component vapor pressure data.
ii
(T) = [1 +(0.48 +1.574
i
0.176
2
i
)(1 T
0.5
r,i
)]
2
(2.4)
Already with the introduction of the generalized -function, a good
description of the vapor pressures especially of hydrocarbons is
obtained. But the description of the densities was only slightly
improved compared to the RedlichKwong equation of state.
2.3. The PengRobinson equation of state
In 1976 Peng and Robinson suggested a slightly different attrac-
tive part (Eq. (2.5)) with a modied generalized -function (Eq.
(2.6)) which leads to slightly improved results for liquid densities
[7].
P =
R T
v b
a(T)
v(v +b) +b(v b)
(2.5)
ii
(T) = [1 +(0.37464 +1.54226
i
0.26992
2
i
)(1 T
0.5
r,i
)]
2
(2.6)
2.4. Mixing rules
For a long time the application of cubic equations of state was
limited to non-polar or slightly polar systems. This limitation was
mainly caused by the classical quadratic mixing rule used for the
attractive parameter a (Eq. (2.7)) [8].
a =
x
i
x
j
a
ij
(2.7)
440 B. Schmid, J. Gmehling / J. of Supercritical Fluids 55 (2010) 438447
Fig. 3. Experimental and calculated VLE data for the systemacetone(1)water(2) at
333.15K SRK+quadratic mixing rule.
where the binary parameter a
ij
is calculated using a combination
rule (Eq. (2.8)) with a binary parameter k
ij
which is tted to exper-
imental data [9].
a
ij
=
a
ii
a
jj
(1 k
ij
) (2.8)
Huron and Vidal [10] tried to combine the advantages of g
E
-models
and equations of state by the development of a so-called g
E
-mixing
rule (Eq. (2.9)) at a reference pressure P
ref
=:
a
bRT
=
i
x
i
a
ii
b
i
RT
+
g
E
/RT
0.6931
(2.9)
The improvements obtained in the description of vaporliquid
equilibria are exemplary shown in Figs. 3 and 4. While for the
SoaveRedlichKwong equation in combination with the classic
mixing rule very poor results are obtained for the polar systemace-
tone (1)water (2), the vaporliquid equilibrium behavior of this
system can be described reliably by using the g
E
-mixing rule.
The use of already published, directly tted interaction param-
eters of the implemented g
E
-models within the g
E
-mixing rules
would save the time-consuming retting procedure. But then the
reference pressure should be at pressures near atmospheric pres-
sure. Therst important stepinthis directionwas donebyMollerup
[11]. He connected the excess Helmholtz energy a
E
and the excess
Gibbs energy g
E
0
at zero pressure introducing the FloryHuggins
Fig. 4. Experimental and calculated VLE data for the systemacetone(1)water(2) at
333.15K SRK+g
E
mixing rule UNIQUAC.
term
i
x
i
lnb/b
i
:
a
E
RT
=
g
E
0
RT
Pv
E
RT
+
i
x
i
ln
b
b
i
(2.10)
The rst order Modied HuronVidal (MHV1) mixing rule at the
reference pressure P
ref
=0 was derived by Michelsen [12] setting v
E
equal to 0:
q
1
a
bRT
i
x
i
a
ii
b
i
RT
=
g
E
0
RT
+
i
x
i
ln
b
b
i
(2.11)
Where q
1
depends on the equation of state.
2.5. The predictive SoaveRedlichKwong group contribution
equation of state
The development of g
E
-mixing rules all of a sudden allowed
the combination of the SoaveRedlichKwong equation of state
with the g
E
-model (group contribution method) UNIFAC [1]. The
result was the predictive SoaveRedlichKwong group contribu-
tion equation of state (PSRK) which was published in 1991 by
Holderbaum and Gmehling [13].
The resulting PSRK g
E
-mixing rule for parameter a at a refer-
ence state of P
ref
=1.01325bar is:
a
bRT
=
i
x
i
a
ii
b
i
RT
+
g
E
0
/RT +
i
x
i
ln b/b
i
0.64663
(2.12)
PSRK allows using the already existing group interaction parame-
ter matrix of the UNIFAC model [14]. Furthermore 30 gases have
been added as additional main groups into the PSRK matrix to
extend the range of applicability to processes, such as absorption
and supercritical extraction.
In PSRK the -function proposed by Mathias and Copeman [15]
was used to describe the vapor pressures as f(T) reliably. While
for reduced temperatures T
r
<1 the polynomial form (Eq. (2.13)) is
used,
ii
(T) = [1 +c
1
(1 T
0.5
r,i
) +c
2
(1 T
0.5
r,i
)
2
+c
3
(1 T
0.5
r,i
)
3
]
2
(2.13)
for T
r
>1 the parameters c
2
and c
3
are set to zero. This leads to the
Soave approach which is given in Eq. (2.14).
ii
(T) = [1 +c
1
(1 T
0.5
r,i
)]
2
(2.14)
The MathiasCopeman parameters c
1
c
3
usually are tted to
experimental vapor pressures. If no vapor pressures data are avail-
able, the MathiasCopeman equation is reduced to the Soave
approach given in Eq. (2.14) and the parameter c
1
can be estimated
from the acentric factor following Eq. (2.15).
c
1
= 0.48 +1.574 1.76
2
(2.15)
Today, PSRK is implemented in most process simulators and is
usedworldwide for a variety of applications. The great advantage of
the PSRKmodel is that all of a sudden the UNIFAC group interaction
parameters can be used at sub- as well as supercritical conditions.
But nevertheless, the PSRK model shows all the weaknesses of
the SRK equation of state and the UNIFAC method. So for example
poor results are obtained for the liquid densities of the pure com-
pounds and the mixtures. Furthermore, the results for asymmetric
systems as well as the prediction of excess enthalpies and activity
coefcients at innite dilution are not satisfying. Furthermore, the
MathiasCopeman-functionusedshows a curvature, whichis not
physically correct at high reduced temperature.
The next step in the development of a universal group con-
tribution equation of state was the systematic removal of these
weaknesses. This was achieved by Ahlers and Gmehling. In 2001
B. Schmid, J. Gmehling / J. of Supercritical Fluids 55 (2010) 438447 441
they published the volume translated group contribution equation
of state VTPR [16].
3. The VTPR group contribution equation of state
The volume translated PengRobinson (VTPR) group con-
tribution equation of state (Eq. (3.1)) is the result of a
consequent improvement of equations of state. It is based on the
PengRobinson equation of state [7], the group contribution model
mod. UNIFAC (Do) [2] and the volume translation introduced by
Peneloux et al. [17]. At the same time improved mixing rules for
the parameters a and b are used.
P =
RT
(v +c b)
a(T)
(v +c)(v +c +b) +b(v +c b)
(3.1)
3.1. Attractive parameter and co-volume
As for the van der Waals equation of state, the attractive param-
eter a
ii
and the co-volume b
ii
for the pure components can directly
be calculated from the critical data following Eq. (3.2) and (3.3).
a
ii
(T) = a
c,ii
i
(T) = 0.45724
R
2
T
2
c,i
P
c,i
i
(T) (3.2)
b
ii
= 0.0778
R T
c,i
P
c,i
(3.3)
The temperature dependency of the attractive parameter is taken
into account by the Twu alpha function.
a
ii
(T) = a
c,i
i
(T) (3.4)
3.2. -Function
With the introduction of the Twu--function [18], the prob-
lems caused by the MathiasCopeman -function were solved. The
Twu--function consists of two different forms. It depends on the
considered state. For a reduced temperature T
r
<1, Twu et al. pro-
posed Eq. (3.5).
i
(T) = T
N
i
(M
i
1)
r,i
exp[L
i
(1 T
N
i
M
i
r,i
)] (3.5)
In this case, the parameters L, M and N are tted to pure com-
ponent vapor pressures and sometimes additionally to liquid heat
capacities, since liquid heat capacities deliver additional informa-
tion about the slope of the vapor pressure curve especially at
lowtemperatures, where the experimental determination of vapor
pressure data is difcult [19]. At supercritical conditions, a gener-
alized form of the -function is suggested, where the value of the
alpha function is calculated with the help of the acentric factor
and generalized L, M and N parameters, which were tted to gas
solubilities of hydrogen and nitrogen in long chain alkanes. After
several tests, we decided to use the subcritical form of the Twu -
function at sub- as well as the supercritical conditions [20]. This
procedure leads to the consistency of the -function and leads to
improved results compared to the application of the generalized
form in case of supercritical conditions.
3.3. Volume translation
To improve the results for liquid densities calculated by cubic
equations of state, Peneloux et al. [17] introduced a constant trans-
lation parameter c. The c-parameter can be obtained from the
volumes calculated with the PengRobinson (PR) EOS and experi-
mental volumes at a reduced temperature of 0.7 (Eq. (3.6)).
c =
i
x
i
c
i
with c
i
= v
i,PR
v
i,exp
(3.6)
Fig. 5. Experimental and calculated densities as a function of temperature using the
PengRobinson equation of state.
Froma comparisonof the results showninFigs. 5 and6 for different
compounds the signicant improvement for liquid densities calcu-
lated by the VTPR EOS (Fig. 6) compared to the PengRobinson EOS
(Fig. 5) can be recognized.
Still more impressive is a comparison with the results of the van
der Waals equation of state. For methanol the results are shown in
Fig. 2 together with the results of other cubic equations of state.
As can be seen fromFig. 2, the experimental and predicted den-
sities using VTPR near the critical point (T
r
>0.8) are not in a very
good agreement. Smaller deviations at high reduced temperatures
could be achieved introducing a temperature dependent transla-
tionparameter. But theintroductionof this temperaturedependent
translation parameter may result in a crossing of the isotherms
at supercritical conditions. Therefore, a temperature independent
volume translation parameter was chosen, which does not show
these effects [13].
3.4. Improved mixing rules
Besides the change in the -function and the introduction of the
concept of volume translation, also improved mixing rules for the
parameter a and b suggested by Chen et al. [21] were introduced.
Fig. 6. Experimental and calculated densities as a function of temperature using the
VTPR equation of state.
442 B. Schmid, J. Gmehling / J. of Supercritical Fluids 55 (2010) 438447
Fig. 7. Experimental [4] and predicted vaporliquid phase equilibrium behavior using PSRK (. . .) and VTPR () of the symmetric system ethane (1)propane (2) and the
asymmetric system ethane (1)dodecane (2).
The new VTPR g
E
-mixing rule for the attractive parameter a (Eq.
(3.7)) nowonlycontains theresidual part of theexcess Gibbs energy
[2] at a reference pressure of 1.01325bar.
a
b
=
i
x
i
a
ii
b
ii
+
g
E
res
0.53087
(P
ref
= 1.01325 bar) (3.7)
The b parameter is calculated following the quadratic mixing rule
shown in Eq. (3.8).
b =
j
x
i
x
j
b
ij
(3.8)
The cross parameter b
ij
is derived from a nonlinear combination
rule with as exponent.
b
3/4
ij
=
b
3/4
ij
+b
3/4
jj
2
(3.9)
The exponent is adopted from the g
E
-model mod. UNIFAC
(Do) where it was introduced to improve the results for asym-
metric systems. In Fig. 7 the predicted VLE data for the systems
ethanepropane and ethanedodecane using PSRK and VTPR are
shown together with the experimental date. While similar good
results are obtained for the system ethanepropane, only VTPR is
able to predict the symmetric and the strong asymmetric system
over awidetemperaturerange, wherebyit has tobementionedthat
no group interaction parameters are required for alkanealkane
systems.
3.5. Group interaction parameters
While for alkanealkane systems no group interaction param-
eters are required, for other combinations group interaction
parameters are tted simultaneously to reliable experimental
phase equilibrium data like VLE,
, LLE, SLE of eutectic systems,
gas solubilities, azeotropic data and excess properties (h
E
, c
E
p
). The
objective function presented in Eq. (3.10) is minimized during the
parameter tting procedure.
F = w
VLE
VLE +w
AZD
AZD+w
h
E h
E
+w
c
E
P
c
E
P
+w
GLE
GLE
+w
+w
SLE
SLE +w
LLE
LLE = Minimum (3.10)
The contribution of the deviation between experimental and calcu-
lated values of the different data types to the objective function can
be adjusted with the help of the weighting factors w
i
. VLE data of
normal and low boiling components are suitable to provide infor-
mation about the real behavior as a function of composition. Excess
enthalpies describe the temperature dependency of the activity
coefcient and can be used as supporting information at high tem-
peratures. Gas solubilities as well as activity coefcients at innite
dilution provide the required information about the real behavior
in the dilute region. SLE data of eutectic systems can be used as
supporting information at low temperatures. Finally, liquidliquid
equilibrium data are often the only available information for sys-
tems with strong deviations from Raoults law.
In the VTPR model for the parameters the same temperature
function as in mod. UNIFAC (Do) is used (Eq. (3.11)). Depending on
the strength of the temperature dependence 26 group interaction
parameters are tted.
nm
= exp
a
nm
+b
nm
T +c
nm
T
2
T
(3.11)
Using the tted group interaction parameters, the VTPR model
can be applied to any kind of systems. In Fig. 8 the predicted results
for several n-alkanealcohol systems using the VTPR group contri-
bution equation of state and modied UNIFAC are shown together
with the experimental data.
It can be seen, that VTPR is able to predict the mixture proper-
ties with the same quality as mod. UNIFAC (Do). But in contrast to
g
E
-models, equations of state can also handle systems with super-
critical compounds, e.g. hexaneethanol at 503.15K. At the same
time other important thermophysical properties, such as densities,
enthalpies etc., can be obtained.
In Fig. 9 the predicted results using the VTPR and PSRK model
are shown together with the experimental results for the binary
system carbon dioxide(1)cyclohexane(2).
It can be seen that besides vaporliquid equilibria also reli-
able results for excess enthalpies are predicted. Even the pressure
dependence of these properties is described correctly.
The predicted VLE behavior using PSRK and VTPR for different
CO
2
n-alkane systems is shown in Fig. 10 together with the exper-
imental data. While the vaporliquid equilibrium behavior of the
symmetric systemCO
2
propane and CO
2
hexane is described reli-
ably with both equations of state, only VTPR is able to describe the
strong asymmetric systems CO
2
eicosane and CO
2
octacosane.
4. Range of applicability
The applicability of the VTPR equation of state is not limited to
the reliable prediction of binary phase equilibria and excess prop-
erties. There are a lot of other elds of application for VTPR, for
example:
the identication of separation problems, e.g. azeotropic points
in multicomponent systems,
B. Schmid, J. Gmehling / J. of Supercritical Fluids 55 (2010) 438447 443
Fig. 8. Experimental and predicted properties using VTPR () and mod. UNFAC (Do) (. . .) for different n-alkanealcohol systems.
the construction of residual curves and borderlines,
the design of separation columns,
the selection of suitable solvents for separation processes, chem-
ical processes,. . .
the selection of working uids for thermodynamic cycles,
the consideration of the real behavior on the chemical equilib-
rium conversion (K
, K
),
the prediction of ash points of ammable liquid mixtures,
the prediction of the fate of a chemical in the environment, bioac-
cumulation effects,. . .
the prediction of various thermodynamic properties (h, h
v
,
s,. . .),
the reliable description of the diffusional mass transfer (using
a
i
, f
i
,
i
instead of c
i
),
. . .
4.1. Identication of separation problems
Because nearly 90% of the separation processes in the chemical
as well as in the oil and gas processing industry are distillation
processes, the knowledge about azeotropic points is of a great
importance. In Table 1, the predicted azeotropic points for the qua-
ternary system carbon dioxide (1)ethane (2)hydrogen sulde
(3)propane (4) at 266.5K are listed and compared with the mean
values stored in the Dortmund Data Bank [4].
As can be seen, the results obtained using the VTPR group con-
tribution equation of state are in a very good agreement with
the experimental data stored in the Dortmund Data Bank. In all
cases azeotropic and non-azeotropic behavior is predicted cor-
rectly. Unfortunately for the ternary and quaternary systems no
information about azeotropic behavior was published.
Fig. 9. VLE and excess enthalpies for the binary systemCO
2
(1)ethane (2) calculated with VTPR () and PSRK (. . .) together with the experimental data taken fromthe DDB
[4].
444 B. Schmid, J. Gmehling / J. of Supercritical Fluids 55 (2010) 438447
Fig. 10. Experimental and predicted vaporliquid equilibrium behavior for different sub- and supercritical CO
2
n-alkane systems using PSRK (. . .) and VTPR ().
4.2. Construction of residual curves and borderlines
For distillation processes, besides the knowledge about
azeotropic points, also information about the residual curves and
borderlines are of interest to fully understand the separation pro-
cess [22].
The predicted residual curves and the border line for the ternary
system CO
2
H
2
SC
2
H
6
with two binary azeotropes is shown in
Fig. 11. As can be seen two distillation regions are obtained.
4.3. Selection of suitable solvent for separation processes
The computer supported selection of a suitable entrainer for a
separation process is an important task in process development. A
procedure for the selection of suitable solvents for azeotropic or
extractive distillation with the help of predictive thermodynamic
models or access to a large factual data bank was introduced by
Gmehling and Mllmann [23]. Using this procedure for the separa-
tion of the azeotropic systemCO
2
ethane important for tertiary oil
recovery higher alkanes are found by this procedure. For propane
as entrainer the predicted result using VTPR are shown in Fig. 12.
As can be seen from Fig. 12 the addition of 80mole% propane
leads to an increasing volatility of carbon dioxide which allows
separating CO
2
ethane by extractive distillation.
4.4. Prediction of the chemical equilibrium conversion
The equilibrium state of a reversible chemical reaction is
described by the equilibrium constant K, which can be calculated
using standard thermodynamic properties.
K = K
x
(3.12)
Following Eq. (3.12), the real behavior has to be taken into account
to be able to calculate the chemical equilibrium conversion.
As example reaction, the acidic catalyzed cleavage reaction of
tert-amyl-methyl-ether (TAME) is shown in Fig. 13. As catalyst,
the macroreticular, sulfonic acid ion-exchange resin Amberlyst 36
from Rohm and Haas was used. The cleavage products are isoamy-
lene (2-methyl-2-butene (2M2B), respectively, 2-methyl-1-butene
(2M1B)) and methanol.
The experimentallydeterminedandpredictedchemical equilib-
rium conversions using VTPR (), modied UNIFAC (Do) (. . .) and
Table 1
Experimental andcalculatedazeotropes using VTPRfor the quaternary systemcarbondioxide (1)ethane (2)hydrogensulde (3)propane (4) at 266.5K(n.a. =not available).
System Predicted using VTPR Experimental
Type P (bar) y
2,az
y
3,az
Type P (bar) y
2,az
y
3,az
12 homPmax 33.36 0.3127 homPmax 33.27 0.33
13 None None
14 None None
23 homPmax 20.59 0.9097 0.0903 homPmax 20.68 0.896 0.104
24 None None
34 homPmax 8.97 0.8301 homPmax n.a. 0.83
123 None n.a.
124 None n.a.
134 None n.a.
234 None n.a.
1234 None n.a.
B. Schmid, J. Gmehling / J. of Supercritical Fluids 55 (2010) 438447 445
Fig. 11. Border lines and residual curve for the ternary system CO
2
-ethane-H
2
S at
266.5K derived from VTPR.
Fig. 12. yx-data for the system CO
2
(1)ethane (2) in the presence of 80mole%
propane at 266.5K and solvent free basis predicted with VTPR.
assuming ideal behavior () for the TAME cleavage in the temper-
ature range from 300K to 370K are shown in Fig. 14.
As can be seen, using the VTPR group contribution equation of
state leads tosimilar goodresults as the groupcontributionmethod
mod. UNIFAC (Do).
In Fig. 15, additionally the experimental [23] and predicted
results for the chemical equilibrium conversion of the tert-
amyl-methyl-ether (TAME) cleavage reaction in the temperature
range from 300K to 370K in the presence of n-pentane (1:1)
as inert solvent are shown together with the experimental
values.
As can be concluded from the results shown above, modi-
ed UNIFAC (Do) and the VTPR equation of state are able to
describe the equilibrium conversion of the TAME cleavage reli-
Fig. 13. Reaction equation of the acidic catalyzed cleavage reaction of tert-amyl-
methyl-ether (TAME) to 2-methyl-2-butene (2M2B), 2-methyl-1-butene (2M1B)
and methanol.
Fig. 14. Experimental ([24], [25], [26]) andcalculatedresults for the chemical
equilibriumconversionof tert-amyl-methyl-ether (TAME) inthe temperature range
from 300K to 370K using VTPR (), mod. UNIFAC (Do) (. . .) and assuming ideal
behavior ().
ably in the temperature range covered. It can be seen that the
dilution effect, which following the LeChatelier principle should
lead to a lower chemical equilibrium conversion is compensated
by the real behavior K
.
4.5. Prediction of miscibility gaps
Liquidliquidequilibria canoccur if a systemshows strong devi-
ation from Raoults law. In separation processes as for example
extraction or hetero-azeotropic distillation processes the concen-
tration differences in the two liquid phases are used for the
separation. For the description of the LLE behavior (distribution
coefcients, binodal curve) often g
E
-models, such as NRTL or
UNIQUAC are applied. But of course also equations of state can
be applied. For the ternary system nitrogen-methane-ethane the
results of the VTPR group contribution equation of state are shown
in Fig. 16 together with the experimental data. It can be seen that at
least qualitative agreement is obtained. Furthermore the predicted
LLE results for the binary system nitrogenethane as a function
of temperature are in good agreement with the experimental
ndings.
Fig. 15. Experimental () and calculated results for the chemical equilibrium con-
version of tert-amyl-methyl-ether (TAME) in the temperature range from 300K
to 370K in the presence of n-pentane (1:1) as inert solvent using VTPR (), mod.
UNIFAC (Do) (. . .) and assuming ideal behavior ().
446 B. Schmid, J. Gmehling / J. of Supercritical Fluids 55 (2010) 438447
Fig. 16. Experimental [4] and predicted LLE data of the ternary system nitrogenmethaneethane at 122K using VTPR.
Fig. 17. Experimental () [4] and predicted solubilities of n-alkanes in water as a
function of temperature using VTPR () and mod. UNIFAC (Do) (. . .).
4.6. Solubility of alkanes in water
For environmental technology the knowledge about the solu-
bility of n-alkanes in water is of special interest, especially for the
design of crude oil processing plants and wastewater treatment.
Usingreliable groupinteractionparameters ttedtoa huge amount
of experimental data [4], the VTPR equation can be successfully
applied for the calculation of alkane solubilities in water.
As can be seen from Fig. 17, the solubility of n-alkanes in water
correlated using the volume translated PengRobinson group con-
tributionequationof state(solidlines) areinagoodagreement with
the experimental data taken fromthe Dortmund Data Bank [4] and
improved to the results derived from the mod. UNIFAC (Do) corre-
lation (dashed lines). For the comparison of both models with the
experimental ndings, the respective groupinteractionparameters
for VTPR as well as mod. UNIFAC (Do) were derived from a corre-
lation of the same data base and are not identical with the group
interaction parameters for the main groups H
2
OCH
2
.
5. Conclusion
Based on the doctoral thesis of Johannes Diderik van der Waals,
a lot of work was spent on the improvement of the cubic equation
of state developed by him. Over the years, most of the weak-
nesses like the poor results for vapor pressures or liquid densities,
strong polar or asymmetric systems could be removed. The pre-
sented VTPR group contribution equation of state is the result
of the consequent improvement of cubic equations of state and
can be applied successfully for process development. No matter
whether the investigated systemis a polar or non-polar multicom-
ponent mixture containing supercritical compounds the volume
translated PengRobinson group contribution equation of state can
be successfully applied. The prediction quality of VTPR is as good
as for modied UNIFAC (Do) and distinctly improved compared to
PSRK. In contrast to modied UNIFAC (Do), VTPR at the same time
allows the prediction of densities, enthalpies, heat capacities and
so on.
List of symbols
a cohesive energy parameter of cubic equations of state
a
E
excess Helmholtz energy
a
nm
group interaction parameter
b co-volume parameter of cubic equations of state
b
nm
group interaction parameter
c translation parameter for the volume translated
PengRobinson equation of state
c
nm
group interaction parameter
c
p
Isobaric heat capacity
F objective function
g molar Gibbs energy
h molar enthalpy
h
v
molar enthalpy of vaporization
h
R
molar enthalpy of reaction
K equilibrium constant
L,M,N TwuBuckCunninghamCoon -function parameters
P pressure
R universal gas constant
s molar entropy
T absolute temperature
v molar volume
w weighting factor
x liquid phase mole fraction
Greek letters
temperature dependent a(T)-function
activity coefcient
acentric factor
fugacity coefcient
temperature function
chemical potential
difference between two values
B. Schmid, J. Gmehling / J. of Supercritical Fluids 55 (2010) 438447 447
Subscripts
c critical point
calc calculated
exp experimental
i,j component i,j
r reduced
Superscripts
E excess
id ideal
m, n main groups m, n
innite
Abbreviations
AZD azeotropic data
GLE gasliquid equilibrium
LLE liquidliquid equilibrium
SLE solidliquid equilibrium
UNIQUAC universal quasi chemical model
VLE vaporliquid equilibrium
VTPR volume translated PengRobinson group contribution
equation of state
Acknowledgements
The authors would like to thank the Arbeitsgemeinschaft
industrieller Forschungsvereinigungen (AiF) for the nancial sup-
port of this work (IGF-Project No. 15345N).
References
[1] A.A. Fredenslund, R.L. Jones, J.M. Prausnitz, Group-contribution estimation of
activity coefcients innonideal liquidmixtures, AmericanInstitute of Chemical
Engineers J. 21 (1975) 10861099.
[2] U. Weidlich, J. Gmehling, A modied UNIFAC model. 1. Prediction of VLE, h
E
,
and
, Industrial and Engineering Chemistry Research 26 (1987) 1372.
[3] J.D. van der Waals, Doctoral Thesis, Leiden, 1873.
[4] Dortmund Data Bank 2010, DDBST GmbH. http://www.ddbst.com.
[5] O. Redlich, J.N.S. Kwong, On the thermodynamics of solutions. V. An equation
of state. Fugacities of gaseous solutions, Chemical Reviews 44 (1949) 233.
[6] G. Soave, Equilibrium constants from a modied RedlichKwong equation of
state, Chemical Engineering Science 27 (1972) 1197.
[7] D.Y. Peng, D.B. Robinson, A new two-constant equation of state, Industrial and
Engineering Chemistry Fundamentals 15 (1976) 59.
[8] O. Noll, Ph.D. Thesis, Oldenburg, 1998.
[9] E.H. Benmekki, T.Y. Kwak, G.A. Mansoori, ACS Symposium Series 329, Van der
Waals Mixing Rules for Cubic Equations of State, American Chemical Society,
Washington, D.C., 1987, pp. 101114.
[10] M.-J. Huron, J. Vidal, New mixing rules in simple equations of state for rep-
resenting vapourliquid equilibria of strongly non-ideal mixtures, Fluid Phase
Equilibria 3 (1979) 255271.
[11] J. Mollerup, A note on the derivation of mixing rules from excess Gibbs energy
models, Fluid Phase Equilibria 25 (1986) 323.
[12] M.L. Michelsen, A modied HuronVidal mixing rule for cubic equations of
state, Fluid Phase Equilibria 60 (1990) 213.
[13] T. Holderbaum, J. Gmehling, PSRK: a group contributionequationof state based
on UNIFAC, Fluid Phase Equilibria 70 (1991) 251265.
[14] H.K. Hansen, P. Rasmussen, A. Fredenslund, M. Schiller, J. Gmehling,
Vaporliquid equilibria by UNIFAC group-contribution. 5. revision and exten-
sion, Industrial and Engineering Chemistry Research 30 (1991) 23522355.
[15] P.M. Mathias, T.W. Copeman, Extension of the PengRobinson equation of state
to complex mixtures: evaluation of the various forms of the local composition
concept, Fluid Phase Equilibria 13 (1983) 91108.
[16] J. Ahlers, J. Gmehling, Development of a universal group contribution equation
of state: I. Prediction of liquid densities for pure compounds with a volume
translated PengRobinson equation of state, Fluid Phase Equilibria 191 (2001)
177188.
[17] A. Peneloux, E. Rauzy, R. Freze, A consistent correction for
RedlichKwongSoave volumes, Fluid Phase Equilibria 8 (1982) 723.
[18] C.H. Twu, J.E. Coon, J.R. Cunningham, A new generalized alpha function for a
cubic equation of state Part 1. PengRobinson equation, Fluid Phase Equilibria
105 (1995) 4959.
[19] A. Diedrichs, J. Rarey, J. Gmehling, Prediction of liquid heat capacities by the
group contribution equation of state VTPR, Fluid Phase Equilibria 248 (2006)
5669.
[20] Private communication.
[21] J. Chen, K. Fischer, J. Gmehling, Modicationof PSRKmixingrules andresults for
vaporliquid equilibria, enthalpy of mixing and activity coefcients at innite
dilution, Fluid Phase Equilibria 200 (2002) 411429.
[22] J.D. Seader, J. Ernest, Henley, Separation Process Principles, Wiley & Sons, New
York, 1997, pp.591-599.
[23] J. Gmehling, C. Mllmann, Synthesis of distillation processes using thermo-
dynamic models and the Dortmund Data Bank, Industrial and Engineering
Chemistry Research 37 (1998) 31123123.
[24] V. Liebert, T. Hector, J. Gmehling, Chemical equilibrium conversion of the tert-
amyl-methyl-ether synthesis in the presence of n-pentane, tetrahydrofuran, or
benzene, Industrial andEngineering Chemistry Research49(2010) 44124419.
[25] L.K. Rihko, J.A. Linnekoski, A.O.I. Krause, Reaction equilibria in the synthesis of
2-methoxy-2-methylbutaneand2-ethoxy-2-methylbutaneintheliquidphase,
J. Chemical and Engineering Data 39 (1994) 700704.
[26] F.H. Syed, C. Egleston, R.J. Datta, Tert-amyl methyl ether (TAME). Thermo-
dynamic analysis of reaction equilibria in the liquid phase, J. Chemical and
Engineering Data 45 (2000) 319323.
También podría gustarte
- Lab 2Documento16 páginasLab 2Gustavo Adolfo RodriguezAún no hay calificaciones
- Theoretical Analysis of Steady States For Ester Hydrolysis in An Enzymatic Membrane Reactor With Product RetentionDocumento11 páginasTheoretical Analysis of Steady States For Ester Hydrolysis in An Enzymatic Membrane Reactor With Product RetentionGustavo Adolfo RodriguezAún no hay calificaciones
- TesisDocumento14 páginasTesisGustavo Adolfo RodriguezAún no hay calificaciones
- 99 05Documento2 páginas99 05Gustavo Adolfo RodriguezAún no hay calificaciones
- JT Coefficient and JT Inversion Cueves For Pure Compounds, FPE, 306,2011,181-189Documento9 páginasJT Coefficient and JT Inversion Cueves For Pure Compounds, FPE, 306,2011,181-189Gustavo Adolfo RodriguezAún no hay calificaciones
- The Method of Lines For The Solutions of SomeDocumento24 páginasThe Method of Lines For The Solutions of SomeGustavo Adolfo RodriguezAún no hay calificaciones
- On Volume Translations in Equations of StateDocumento9 páginasOn Volume Translations in Equations of StateGustavo Adolfo RodriguezAún no hay calificaciones
- Equation of StateDocumento6 páginasEquation of StateJanardhan CnAún no hay calificaciones
- Further Development of Modified UNIFAC (Dortmund), Revision and Extension 5.Documento10 páginasFurther Development of Modified UNIFAC (Dortmund), Revision and Extension 5.Gustavo Adolfo RodriguezAún no hay calificaciones
- Wagner Equation Constants for Vapour Pressure PredictionDocumento17 páginasWagner Equation Constants for Vapour Pressure PredictionGustavo Adolfo RodriguezAún no hay calificaciones
- Production EstimatesDocumento6 páginasProduction EstimatesGustavo Adolfo RodriguezAún no hay calificaciones
- Setaram 2008228105941795445Documento8 páginasSetaram 2008228105941795445Gustavo Adolfo RodriguezAún no hay calificaciones
- A Procedure For The Calculation of The Natural Gas Molar Heat CapacityDocumento9 páginasA Procedure For The Calculation of The Natural Gas Molar Heat Capacityjlg314Aún no hay calificaciones
- On The Classification of Phase Transformation, SM, 46,2002,893-898Documento6 páginasOn The Classification of Phase Transformation, SM, 46,2002,893-898Gustavo Adolfo RodriguezAún no hay calificaciones
- Setaram 2008228105941795445Documento8 páginasSetaram 2008228105941795445Gustavo Adolfo RodriguezAún no hay calificaciones
- A High Area of Rock With A Very Steep Side. Lesson 268 Pronunciation HDocumento1 páginaA High Area of Rock With A Very Steep Side. Lesson 268 Pronunciation HGustavo Adolfo RodriguezAún no hay calificaciones
- First-Year University Chemistry Textbooks' Misrepresentation of Gibbs EnergyDocumento7 páginasFirst-Year University Chemistry Textbooks' Misrepresentation of Gibbs EnergyGustavo Adolfo RodriguezAún no hay calificaciones
- 1 s2.0 S1003995309600815 MainDocumento4 páginas1 s2.0 S1003995309600815 MainAdeyi SegunAún no hay calificaciones
- Andreas Handbook BF2Documento44 páginasAndreas Handbook BF2Setiawan EkoAún no hay calificaciones
- Calorimetric Study of The Entropy Relation in The NaCl-KCl SystemDocumento5 páginasCalorimetric Study of The Entropy Relation in The NaCl-KCl SystemGustavo Adolfo RodriguezAún no hay calificaciones
- Estimation of The Vaporization Heat - Fpe, 313,2012,91-96Documento6 páginasEstimation of The Vaporization Heat - Fpe, 313,2012,91-96Gustavo Adolfo RodriguezAún no hay calificaciones
- Unifed Correlation For Estimating Specific Chemical Exergy of Solid and Liquid Fuels.E, 40,2012,164-173Documento10 páginasUnifed Correlation For Estimating Specific Chemical Exergy of Solid and Liquid Fuels.E, 40,2012,164-173Gustavo Adolfo RodriguezAún no hay calificaciones
- Supplementary Vapo Pressue Data JCHT, 53,2012,114-118Documento5 páginasSupplementary Vapo Pressue Data JCHT, 53,2012,114-118Gustavo Adolfo RodriguezAún no hay calificaciones
- Nuevo Documento de Texto EnriquecidoDocumento1 páginaNuevo Documento de Texto EnriquecidoGustavo Adolfo RodriguezAún no hay calificaciones
- An Empirical Equation For Temperature and Pressure Dependence of Liquid Heat Capacity - Jtche, 40,2009,105-109Documento5 páginasAn Empirical Equation For Temperature and Pressure Dependence of Liquid Heat Capacity - Jtche, 40,2009,105-109Gustavo Adolfo RodriguezAún no hay calificaciones
- Applied Calculus Math 215: Karl Heinz DovermannDocumento302 páginasApplied Calculus Math 215: Karl Heinz DovermannthanananAún no hay calificaciones
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeCalificación: 4 de 5 estrellas4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)De EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Calificación: 4 de 5 estrellas4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe EverandNever Split the Difference: Negotiating As If Your Life Depended On ItCalificación: 4.5 de 5 estrellas4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeDe EverandShoe Dog: A Memoir by the Creator of NikeCalificación: 4.5 de 5 estrellas4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDe EverandThe Emperor of All Maladies: A Biography of CancerCalificación: 4.5 de 5 estrellas4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceCalificación: 4 de 5 estrellas4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe EverandThe Little Book of Hygge: Danish Secrets to Happy LivingCalificación: 3.5 de 5 estrellas3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnDe EverandTeam of Rivals: The Political Genius of Abraham LincolnCalificación: 4.5 de 5 estrellas4.5/5 (234)
- Grit: The Power of Passion and PerseveranceDe EverandGrit: The Power of Passion and PerseveranceCalificación: 4 de 5 estrellas4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaCalificación: 4.5 de 5 estrellas4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryCalificación: 3.5 de 5 estrellas3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealDe EverandOn Fire: The (Burning) Case for a Green New DealCalificación: 4 de 5 estrellas4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureCalificación: 4.5 de 5 estrellas4.5/5 (474)
- Rise of ISIS: A Threat We Can't IgnoreDe EverandRise of ISIS: A Threat We Can't IgnoreCalificación: 3.5 de 5 estrellas3.5/5 (137)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersCalificación: 4.5 de 5 estrellas4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaDe EverandThe Unwinding: An Inner History of the New AmericaCalificación: 4 de 5 estrellas4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyCalificación: 3.5 de 5 estrellas3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreCalificación: 4 de 5 estrellas4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Calificación: 4.5 de 5 estrellas4.5/5 (119)
- The Perks of Being a WallflowerDe EverandThe Perks of Being a WallflowerCalificación: 4.5 de 5 estrellas4.5/5 (2099)
- Her Body and Other Parties: StoriesDe EverandHer Body and Other Parties: StoriesCalificación: 4 de 5 estrellas4/5 (821)
- Tutorial C5 AnswerDocumento7 páginasTutorial C5 AnswerAustin Phua Yun HockAún no hay calificaciones
- Chapter 6 PDFDocumento56 páginasChapter 6 PDFhamza abbasAún no hay calificaciones
- Circular Motion Test PDF - 1539691471832 - QISNtDocumento1 páginaCircular Motion Test PDF - 1539691471832 - QISNtSathya NarayananAún no hay calificaciones
- Energy Conservation Explained in 40 CharactersDocumento17 páginasEnergy Conservation Explained in 40 Charactersozgurturunc4Aún no hay calificaciones
- Properties of Pure Substances: Çengel BolesDocumento34 páginasProperties of Pure Substances: Çengel Boleskebaman1986Aún no hay calificaciones
- Modeling gas formative assessmentDocumento3 páginasModeling gas formative assessmentPaula AstudilloAún no hay calificaciones
- Q No. AnsDocumento19 páginasQ No. AnsPankaj SinghAún no hay calificaciones
- 1965 Diagram Technique For Nonequilibrium Processes KeldyshKeldyshDocumento9 páginas1965 Diagram Technique For Nonequilibrium Processes KeldyshKeldyshvlava89Aún no hay calificaciones
- SPONTANEITY, ENTROPY AND FREE ENERGY CHANGESDocumento32 páginasSPONTANEITY, ENTROPY AND FREE ENERGY CHANGESNitya DewiAún no hay calificaciones
- Quantum Mechanics I Course at Shiv Nadar UniversityDocumento3 páginasQuantum Mechanics I Course at Shiv Nadar UniversityAnuprava BokshiAún no hay calificaciones
- Ideal Gas Equation SheetDocumento1 páginaIdeal Gas Equation SheetIsaac HanssenAún no hay calificaciones
- Energy Analysis of Closed Systems Study Guide in Powerpoint: To AccompanyDocumento68 páginasEnergy Analysis of Closed Systems Study Guide in Powerpoint: To AccompanyChuck NorrisAún no hay calificaciones
- FSC Physics 2 Year: Chapter 15: Electromagnetic InductionDocumento15 páginasFSC Physics 2 Year: Chapter 15: Electromagnetic InductionAdeela UmarAún no hay calificaciones
- Flannery PDFDocumento8 páginasFlannery PDFzoehdiismailAún no hay calificaciones
- Study Guide NPT220 (3 July 2017)Documento12 páginasStudy Guide NPT220 (3 July 2017)Blessed MuyangaAún no hay calificaciones
- IJREAMV05I0351087Documento5 páginasIJREAMV05I0351087Dang AntheaAún no hay calificaciones
- Entropy Balance: Prof. Dr. Uğur AtikolDocumento14 páginasEntropy Balance: Prof. Dr. Uğur AtikolRajesh ShuklaAún no hay calificaciones
- Line and Surface Integrals ChapterDocumento28 páginasLine and Surface Integrals ChapterDinh LâmAún no hay calificaciones
- Applied Physics Syllabus BreakdownDocumento42 páginasApplied Physics Syllabus BreakdownDhruv GoyalAún no hay calificaciones
- Basic Concepts 3: Topics in Contemporary PhysicsDocumento32 páginasBasic Concepts 3: Topics in Contemporary PhysicsAndy HoAún no hay calificaciones
- HermiteCurvesBezierCurvesExamplesDocumento5 páginasHermiteCurvesBezierCurvesExampleskhalil alhatabAún no hay calificaciones
- Chap 10Documento31 páginasChap 10bettieboomAún no hay calificaciones
- Thermodynamics: Schaum'S Solved Problems SeriesDocumento3 páginasThermodynamics: Schaum'S Solved Problems SeriesMichael West50% (2)
- PWE 26-2 - Compton ScatteringDocumento1 páginaPWE 26-2 - Compton ScatteringmulatAún no hay calificaciones
- Quantum Hall Effect - BDocumento7 páginasQuantum Hall Effect - Bspow123Aún no hay calificaciones
- Hw4 137BDocumento3 páginasHw4 137Btadf2Aún no hay calificaciones
- Textbook of Heat Transfer - Sukhatme, S. P.Documento122 páginasTextbook of Heat Transfer - Sukhatme, S. P.Chaitanya Potti67% (3)
- BME - Module 1Documento103 páginasBME - Module 1Pranav JayasuryaAún no hay calificaciones
- Previous Hse Questions and Answers of The Chapter "Thermodynamics"Documento8 páginasPrevious Hse Questions and Answers of The Chapter "Thermodynamics"Muhammed SadiqAún no hay calificaciones