Documentos de Académico
Documentos de Profesional
Documentos de Cultura
Signs and Symptoms
Cargado por
Galuh NurfadillahDescripción original:
Título original
Derechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Signs and Symptoms
Cargado por
Galuh NurfadillahCopyright:
Formatos disponibles
Lazoff M. meningitis. Editor-in-Chief, Medical Computing Review. Update Feb 2, 2010.
Available at.http://emedicine.medscape.com/article/784389.
Meningitis is a clinical syndrome characterized by inflammation of the meninges.
Signs and symptoms
The classic triad of bacterial meningitis consists of the following:
Fever
Headache
Neck stiffness
Other symptoms can include nausea, vomiting, photalgia (photophobia), sleepiness,
confusion, irritability, delirium, and coma. Patients with viral meningitis may have a
history of preceding systemic symptoms (eg, myalgias, fatigue, or anorexia).
The history should also address the following:
Epidemiologic factors and predisposing risks
Exposure to a patients or animals with a similar illness
Previous medical treatment and existing conditions
Geographic location and travel history
Season and temperature
Acute bacterial meningitis in otherwise healthy patients who are not at the extremes
of age presents in a clinically obvious fashion; however, subacute bacterial
meningitis often poses a diagnostic challenge.
General physical findings in viral meningitis are common to all causative agents.
Enteroviral infection is suggested by the following:
Exanthemas
Symptoms of pericarditis, myocarditis, or conjunctivitis
Syndromes of pleurodynia, herpangina, and hand-foot-and-mouth disease
Infants may have the following:
Bulging fontanelle (if euvolemic)
Paradoxic irritability (ie, remaining quiet when stationary and crying when held)
High-pitched cry
Hypotonia
The examination should evaluate the following:
Focal neurologic signs
Signs of meningeal irritation
Systemic and extracranial findings
Chronic meningitis
In chronic meningitis, it is essential to perform careful general, systemic, and
neurologic examinations, looking especially for the following:
Lymphadenopathy
Papilledema and tuberculomas during funduscopy
Meningismus
Cranial nerve palsies
Patients with aseptic meningitis syndrome usually appear clinically nontoxic, with no
vascular instability. They characteristically have an acute onset of meningeal
symptoms, fever, and CSF pleocytosis that is usually prominently lymphocytic.
See Clinical Presentation for more detail.
Diagnosis
The diagnostic challenges in patients with clinical findings of meningitis are as
follows:
Early identification and treatment of patients with acute bacterial meningitis
Assessing whether a treatable CNS infection is present in those with suspected
subacute or chronic meningitis
Identifying the causative organism
Blood studies that may be useful include the following:
Complete blood count (CBC) with differential
Serum electrolytes
Serum glucose (which is compared with the CSF glucose)
Blood urea nitrogen (BUN) or creatinine and liver profile
In addition, the following tests may be ordered:
Blood, nasopharynx, respiratory secretion, urine or skin lesion cultures
Syphilis testing
Serum procalcitonin testing
Lumbar puncture and CSF analysis
Neuroimaging (CT of the head and MRI of the brain)
See Workup for more detail.
Management
Initial measures include the following:
Shock or hypotension Crystalloids
Altered mental status Seizure precautions and treatment (if necessary), along
with airway protection (if warranted)
Stable with normal vital signs Oxygen, IV access, and rapid transport to the
emergency department (ED)
Treatment of bacterial meningitis includes the following:
Prompt initiation of empiric antibacterial therapy as appropriate for patient age and
condition
After identification of the pathogen and determination of susceptibilities, targeted
antibiotic therapy as appropriate for patient age and condition
Steroid (typically, dexamethasone) therapy
In patients with nosocomial meningitis, intrathecal antibiotics
The following systemic complications of acute bacterial meningitis must be treated:
Hypotension or shock
Hypoxemia
Hyponatremia
Cardiac arrhythmias and ischemia
Stroke
Exacerbation of chronic diseases
Most cases of viral meningitis are benign and self-limited, but in certain instances,
specific antiviral therapy may be indicated, if available.
Other types of meningitis are treated with specific therapy as appropriate for the
causative pathogen, as follows:
Fungal meningitis - Cryptococcal (amphotericin B, flucytosine, fluconazole,
itraconazole), Coccidioides immitis (fluconazole, intrathecal amphoytericin B,
itraconazole), Histoplasma capsulatum (liposomal amphotericin B, itraconazole),
or Candida (IM or aqueous penicillin G, probenecid)
Tuberculous meningitis (isoniazid, rifampin, pyrazinamide, ethambutol,
streptomycin)
Parasitic meningitis Amebic (amphotericin B, miconazole, rifampin) or helminthic
(largely supportive)
Lyme meningitis (ceftriaxone; alternatively, penicillin G, doxycycline,
chloramphenicol)
See Treatment and Medication for more detail.
Image library
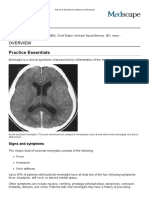
Acute bacterial meningitis. This axial nonenhanced
computed tomography scan shows mild ventriculomegaly and sulcal effacement
Background
Infections of the central nervous system (CNS) can be divided into 2 broad
categories: those primarily involving the meninges (meningitis; see the image below)
and those primarily confined to the parenchyma (encephalitis).
Pneumococcal meningitis in a patient with alcoholism. Courtesy
of the CDC/Dr. Edwin P. Ewing, Jr.
Meningitis is a clinical syndrome characterized by inflammation of the meninges, the
3 layers of membranes that enclose the brain and spinal cord. These layers consist
of the following:
Dura - A tough outer membrane
Arachnoid - A lacy, weblike middle membrane
Subarachnoid space - A delicate, fibrous inner layer that contains many of the
blood vessels that feed the brain and spinal cord
Risk factors for meningitis include the following:
Extremes of age (< 5 or >60 years)
Diabetes mellitus, renal or adrenal insufficiency, hypoparathyroidism, or cystic
fibrosis
Immunosuppression, which increases the risk of opportunistic infections and acute
bacterial meningitis
HIV infection, which predisposes to bacterial meningitis caused by encapsulated
organisms, primarily Streptococcus pneumoniae, and opportunistic pathogens
Crowding (such as that experienced by military recruits and college dorm
residents), which increases the risk of outbreaks of meningococcal meningitis
Splenectomy and sickle cell disease, which increase the risk of meningitis
secondary to encapsulated organisms
Alcoholism and cirrhosis
Recent exposure to others with meningitis, with or without prophylaxis
Contiguous infection (eg, sinusitis)
Dural defect (eg, traumatic, surgical, or congenital)
Thalassemia major
Intravenous (IV) drug abuse
Bacterial endocarditis
Ventriculoperitoneal shunt
Malignancy (increased risk of Listeria infection)
Some cranial congenital deformities
Clinically, meningitis manifests with meningeal symptoms (eg, headache, nuchal
rigidity, or photophobia), as well as pleocytosis (an increased number of white blood
cells [WBCs]) in the cerebrospinal fluid (CSF). Depending on the duration of
symptoms, meningitis may be classified as acute or chronic. (See Etiology and
Presentation.)
Anatomically, meningitis can be divided into inflammation of the dura (sometimes
referred to as pachymeningitis), which is less common, and leptomeningitis, which is
more common and is defined as inflammation of the arachnoid tissue and
subarachnoid space. (See Anatomy.)
Meningitis can also be divided into the following 3 general categories:
Bacterial (pyogenic)
Granulomatous
Aseptic
The most common cause of meningeal inflammation is irritation caused by bacterial
or viral infections. The organisms usually enter the meninges through the
bloodstream from other parts of the body. Most cases of bacterial meningitis are
localized over the dorsum of the brain; however, under certain conditions, meningitis
may be concentrated at the base of the brain, as with fungal diseases and
tuberculosis. (See Etiology.)
Bacterial meningitis consists of pyogenic inflammation of the meninges and the
underlying subarachnoid CSF. If not treated, it may lead to lifelong disability or
death.
[1, 2]
Before the antimicrobial era, bacterial meningitis was uniformly fatal, but
with the advent of antimicrobial therapy, the overall mortality from this disease has
decreased. Nonetheless, it remains alarmingly high: approximately 25%. (See
Epidemiology.)
The emergence of resistant bacterial strains has prompted changes in antibiotic
protocols in some countries, including the United States. Apart from dexamethasone,
neuronal cell protectants still hold only future promise as adjunctive therapy. (See
Treatment and Medication.)
The specific infectious agents that are involved in bacterial meningitis vary among
different patient age groups, and the inflammation may evolve into the following
conditions:
Ventriculitis
Empyema
Cerebritis
Abscess formation
Meningitis can also be also classified more specifically according to its etiology.
Numerous infectious and noninfectious causes of meningitis have been identified.
Examples of common noninfectious causes include medications (eg, nonsteroidal
anti-inflammatory drugs [NSAIDs] and antibiotics) and carcinomatosis. (See
Etiology.)
Bacterial meningitis
Acute bacterial meningitis denotes a bacterial cause of this syndrome. This is usually
characterized by an acute onset of meningeal symptoms and neutrophilic
pleocytosis. Depending on the specific bacterial cause, the syndrome may be called,
for example, any of the following:
Pneumococcal meningitis
Haemophilus influenzae meningitis
Staphylococcal meningitis
Meningococcal meningitis
Tuberculous meningitis
Pediatric bacterial meningitis
Chronic meningitis is a constellation of signs and symptoms of meningeal irritation
associated with CSF pleocytosis that persists for longer than 4 weeks.
Unlike subacute (developing over 1-7 days) or chronic (>7 days) meningitis, which
have myriad infectious and noninfectious etiologies, acute meningitis (< 1 day) is
almost always a bacterial infection caused by 1 of several organisms. Depending on
age and general condition, these gravely ill patients present acutely with signs and
symptoms of meningeal inflammation and systemic infection of less than 24 hours
(and usually >12 hours) duration.
Patients with acute bacterial meningitis may decompensate very quickly.
Consequently, they require emergency care, including the administration of
appropriate antimicrobial therapy as soon as possible once bacterial meningitis is
suspected or proven.
Nonbacterial meningitis
Fungal and parasitic forms of meningitis are also named according to their specific
etiologic agent (eg, cryptococcal meningitis, Histoplasma meningitis, and amebic
meningoencephalitis).
In many cases, a cause of meningitis is not apparent after initial evaluation, and the
disease is therefore classified as aseptic meningitis. These patients characteristically
have an acute onset of meningeal symptoms, fever, and CSF pleocytosis that is
usually prominently lymphocytic.
When the cause of aseptic meningitis is discovered, the disease can be reclassified
according to its etiology. If appropriate diagnostic methods are performed, a specific
viral etiology is identified in 55-70% of cases of aseptic meningitis. However, the
condition can also be caused by bacterial, fungal, mycobacterial, and parasitic
agents.
If, after an extensive workup, aseptic meningitis is found to have a viral etiology, it
can be reclassified as a form of acute viral meningitis (eg, enteroviral meningitis or
herpes simplex virus [HSV] meningitis).
Pathophysiology
Most cases of meningitis are caused by an infectious agent that has colonized or
established a localized infection elsewhere in the host. Potential sites of colonization
or infection include the skin, the nasopharynx, the respiratory tract, the
gastrointestinal (GI) tract, and the genitourinary tract. The organism invades the
submucosa at these sites by circumventing host defenses (eg, physical barriers,
local immunity, and phagocytes or macrophages).
An infectious agent (ie, a bacterium, virus, fungus, or parasite) can gain access to
the CNS and cause meningeal disease via any of the 3 following major pathways:
Invasion of the bloodstream (ie, bacteremia, viremia, fungemia, or parasitemia)
and subsequent hematogenous seeding of the CNS
A retrograde neuronal (eg, olfactory and peripheral nerves) pathway (eg,Naegleria
fowleri or Gnathostoma spinigerum)
Direct contiguous spread (eg, sinusitis, otitis media, congenital malformations,
trauma, or direct inoculation during intracranial manipulation)
Invasion of the bloodstream and subsequent seeding is the most common mode of
spread for most agents. This pathway is characteristic of meningococcal,
cryptococcal, syphilitic, and pneumococcal meningitis.
Rarely, meningitis arises from invasion via septic thrombi or osteomyelitic erosion
from infected contiguous structures. Meningeal seeding may also occur with a direct
bacterial inoculate during trauma, neurosurgery, or instrumentation. Meningitis in the
newborn may be transmitted vertically, involving pathogens that have colonized the
maternal intestinal or genital tract, or horizontally, from nursery personnel or
caregivers at home.
Local extension from contiguous extracerebral infection (eg, otitis media, mastoiditis,
or sinusitis) is a common cause. Possible pathways for the migration of pathogens
from the middle ear to the meninges include the following:
The bloodstream
Preformed tissue planes (eg, posterior fossa)
Temporal bone fractures
The oval or round window membranes of the labyrinths
The brain is naturally protected from the bodys immune system by the barrier that
the meninges create between the bloodstream and the brain. Normally, this
protection is an advantage because the barrier prevents the immune system from
attacking the brain. However, in meningitis, the blood-brain barrier can become
disrupted; once bacteria or other organisms have found their way to the brain, they
are somewhat isolated from the immune system and can spread.
When the body tries to fight the infection, the problem can worsen; blood vessels
become leaky and allow fluid, WBCs, and other infection-fighting particles to enter
the meninges and brain. This process, in turn, causes brain swelling and can
eventually result in decreasing blood flow to parts of the brain, worsening the
symptoms of infection.
[3]
Depending on the severity of bacterial meningitis, the inflammatory process may
remain confined to the subarachnoid space. In less severe forms, the pial barrier is
not penetrated, and the underlying parenchyma remains intact. However, in more
severe forms of bacterial meningitis, the pial barrier is breached, and the underlying
parenchyma is invaded by the inflammatory process. Thus, bacterial meningitis may
lead to widespread cortical destruction, particularly when left untreated.
Replicating bacteria, increasing numbers of inflammatory cells, cytokine-induced
disruptions in membrane transport, and increased vascular and membrane
permeability perpetuate the infectious process in bacterial meningitis. These
processes account for the characteristic changes in CSF cell count, pH, lactate,
protein, and glucose in patients with this disease.
Exudates extend throughout the CSF, particularly to the basal cisterns, resulting in
the following:
Damage to cranial nerves (eg, cranial nerve VIII, with resultant hearing loss)
Obliteration of CSF pathways (causing obstructive hydrocephalus)
Induction of vasculitis and thrombophlebitis (causing local brain ischemia)
Intracranial pressure and cerebral fluid
One complication of meningitis is the development of increased intracranial pressure
(ICP). The pathophysiology of this complication is complex and may involve many
proinflammatory molecules as well as mechanical elements. Interstitial edema
(secondary to obstruction of CSF flow, as in hydrocephalus), cytotoxic edema
(swelling of cellular elements of the brain through the release of toxic factors from
the bacteria and neutrophils), and vasogenic edema (increased blood brain barrier
permeability) are all thought to play a role.
Without medical intervention, the cycle of decreasing CSF, worsening cerebral
edema, and increasing ICP proceeds unchecked. Ongoing endothelial injury may
result in vasospasm and thrombosis, further compromising CSF, and may lead to
stenosis of large and small vessels. Systemic hypotension (septic shock) also may
impair CSF, and the patient soon dies as a consequence of systemic complications
or diffuse CNS ischemic injury.
Cerebral edema
The increased CSF viscosity resulting from the influx of plasma components into the
subarachnoid space and diminished venous outflow lead to interstitial edema. The
accumulation of the products of bacterial degradation, neutrophils, and other cellular
activation leads to cytotoxic edema.
The ensuing cerebral edema (ie, vasogenic, cytotoxic, and interstitial) significantly
contributes to intracranial hypertension and a consequent decrease in cerebral blood
flow. Anaerobic metabolism ensues, which contributes to increased lactate
concentration and hypoglycorrhachia. In addition, hypoglycorrhachia results from
decreased glucose transport into the spinal fluid compartment. Eventually, if this
uncontrolled process is not modulated by effective treatment, transient neuronal
dysfunction or permanent neuronal injury results.
Cytokines and secondary mediators in bacterial meningitis
Key advances in understanding the pathophysiology of meningitis include insight into
the pivotal roles of cytokines (eg, tumor necrosis factor alpha [TNF-] and interleukin
[IL]-1), chemokines (IL-8), and other proinflammatory molecules in the pathogenesis
of pleocytosis and neuronal damage during occurrences of bacterial meningitis.
Increased CSF concentrations of TNF-, IL-1, IL-6, and IL-8 are characteristic
findings in patients with bacterial meningitis. Cytokine levels, including those of IL-6,
TNF-, and interferon gamma, have been found to be elevated in patients with
aseptic meningitis.
The proposed events involving these inflammation mediators in bacterial meningitis
begin with the exposure of cells (eg, endothelial cells, leukocytes, microglia,
astrocytes, and meningeal macrophages) to bacterial products released during
replication and death; this exposure incites the synthesis of cytokines and
proinflammatory mediators. This process is likely initiated by the ligation of the
bacterial components (eg, peptidoglycan and lipopolysaccharide) to pattern-
recognition receptors, such as the Toll-like receptors (TLRs).
TNF- and IL-1 are most prominent among the cytokines that mediate this
inflammatory cascade. TNF- is a glycoprotein derived from activated monocyte-
macrophages, lymphocytes, astrocytes, and microglial cells.
IL-1, previously known as endogenous pyrogen, is also produced primarily by
activated mononuclear phagocytes and is responsible for the induction of fever
during bacterial infections. Both IL-1 and TNF- have been detected in the CSF of
individuals with bacterial meningitis. In experimental models of meningitis, they
appear early during the course of disease and have been detected within 30-45
minutes of intracisternal endotoxin inoculation.
Many secondary mediators, such as IL-6, IL-8, nitric oxide, prostaglandins (eg,
prostaglandin E2 [PGE2]), and platelet activation factor (PAF), are presumed to
amplify this inflammatory event, either synergistically or independently. IL-6 induces
acute-phase reactants in response to bacterial infection. The chemokine IL-8
mediates neutrophil chemoattractant responses induced by TNF- and IL-1.
Nitric oxide is a free radical molecule that can induce cytotoxicity when produced in
high amounts. PGE2, a product of cyclooxygenase (COX), appears to participate in
the induction of increased blood-brain barrier permeability. PAF, with its myriad
biologic activities, is believed to mediate the formation of thrombi and the activation
of clotting factors within the vasculature. However, the precise roles of all these
secondary mediators in meningeal inflammation remain unclear.
The net result of the above processes is vascular endothelial injury and increased
blood-brain barrier permeability, leading to the entry of many blood components into
the subarachnoid space. In many cases, this contributes to vasogenic edema and
elevated CSF protein levels. In response to the cytokines and chemotactic
molecules, neutrophils migrate from the bloodstream and penetrate the damaged
blood-brain barrier, producing the profound neutrophilic pleocytosis characteristic of
bacterial meningitis.
Genetic predisposition to inflammatory response
The inflammatory response and the release of proinflammatory mediators are critical
to the recruitment of excess neutrophils to the subarachnoid space. These activated
neutrophils release cytotoxic agents, including oxidants and metalloproteins that
cause collateral damage to brain tissue.
Pattern recognition receptors, of which TLR A4 (TLRA4) is the best studied, lead to
increase in the myeloid differentiation 88 (MyD88)-dependent pathway and excess
production of proinflammatory mediators. At present, dexamethasone is used to
decrease the effects of cellular toxicity by neutrophils after they are present.
Researchers are actively seeking ways of inhibiting TLRA4 and other
proinflammatory recognition receptors through genetically engineered suppressors.
[4]
Bacterial seeding
Bacterial seeding of the meninges usually occurs through hematogenous spread. In
patients without an identifiable source of infection, local tissue and bloodstream
invasion by bacteria that have colonized the nasopharynx may be a common source.
Many meningitis-causing bacteria are carried in the nose and throat, often
asymptomatically. Most meningeal pathogens are transmitted through the respiratory
route, including Neisseria meningitidis (meningococcus) and S
pneumoniae (pneumococcus).
Certain respiratory viruses are thought to enhance the entry of bacterial agents into
the intravascular compartment, presumably by damaging mucosal defenses. Once in
the bloodstream, the infectious agent must escape immune surveillance (eg,
antibodies, complement-mediated bacterial killing, and neutrophil phagocytosis).
Subsequently, hematogenous seeding into distant sites, including the CNS, occurs.
The specific pathophysiologic mechanisms by which the infectious agents gain
access to the subarachnoid space remain unclear. Once inside the CNS, the
infectious agents likely survive because host defenses (eg, immunoglobulins,
neutrophils, and complement components) appear to be limited in this body
compartment. The presence and replication of infectious agents remain uncontrolled
and incite the cascade of meningeal inflammation described above.
Etiology
Causes of meningitis include bacteria, viruses, fungi, parasites, and drugs (eg,
NSAIDs, metronidazole, and IV immunoglobulin [IVIg]). Certain risk factors are
associated with particular pathogens.
HIV infection increases susceptibility to meningitis from a variety of pathogens,
including cryptococci, Mycobacterium tuberculosis, syphilis, and Listeria species. In
addition, HIV itself may cause aseptic meningitis (see Meningitis in HIV).
Other viral causes of meningitis include the following:
Enteroviruses
West Nile virus
Human herpesvirus (HHV)-2
Lymphocytic choriomeningitis virus (LCM)
In patients who have had trauma or neurosurgery, the most common
microorganisms are S pneumoniae (if CSF leak is present), Staphylococcus
aureus, enterobacteria, and Pseudomonas aeruginosa. In patients with an infected
ventriculoperitoneal (atrial) shunt, the most common microorganisms
areStaphylococcus epidermidis, S aureus, enterobacteria, Propionibacterium
acnes,and diphtheroids (rare). Consultation with a neurosurgeon is indicated; early
shunt removal is usually necessary for cure.
Pachymeningitis
As indicated by the presence of abundant pus, pachymeningitis most often results
from a bacterial infection (usually staphylococcal or streptococcal) that is localized to
the dura. The organisms most often gain access to the meninges via a skull defect
(eg, a skull fracture) or spread from an infection of the paranasal sinuses or cranial
osteomyelitis.
Haemophilus influenzae meningitis
H influenzae is a small, pleomorphic, gram-negative coccobacillus that is frequently
found as part of the normal flora in the upper respiratory tract. The organism can
spread from one individual to another in airborne droplets or by direct contact with
secretions. Meningitis is the most serious acute manifestation of systemic infection
with H influenzae. (See Haemophilus Meningitis.)
In the past, H influenzae was a major cause of meningitis, and the encapsulated type
b strain of the organism (Hib) accounted for the majority of cases. Since the
introduction of Hib vaccine in the United States in 1990, the overall incidence of H
influenzae meningitis has decreased by 35%, with Hib accounting for fewer than
9.4% of H influenzae cases.
[5]
The isolation of H influenzae in adults suggests the presence of an underlying
medical disorder, such as the following:
Paranasal sinusitis
Otitis media
Alcoholism
CSF leak after head trauma
Functional or anatomic asplenia
Hypogammaglobulinemia
Pneumococcal meningitis
S pneumoniae, a gram-positive coccus, is the most common bacterial cause of
meningitis. In addition, it is the most common bacterial agent in meningitis
associated with basilar skull fracture and CSF leak. It may be associated with other
focal infections, such as pneumonia, sinusitis, or endocarditis (as, for example, in
Austrian syndrome, which is the triad of pneumococcal meningitis, endocarditis, and
pneumonia).
S pneumoniae is a common colonizer of the human nasopharynx; it is present in 5-
10% of healthy adults and 20-40% of healthy children. It causes meningitis by
escaping local host defenses and phagocytic mechanisms, either through choroid
plexus seeding from bacteremia or through direct extension from sinusitis or otitis
media.
Patients with the following conditions are at increased risk for S
pneumoniaemeningitis:
Hyposplenism
Hypogammaglobulinemia
Multiple myeloma
Glucocorticoid treatment
Defective complement (C1-C4)
Diabetes mellitus
Renal insufficiency
Alcoholism
Malnutrition
Chronic liver disease
Streptococcus agalactiae meningitis
Streptococcus agalactiae (group B streptococcus [GBS]) is a gram-positive coccus
that inhabits the lower GI tract. It also colonizes the female genital tract at a rate of 5-
40%, which explains why it is the most common agent of neonatal meningitis
(associated with 70% of cases).
Predisposing risks in adults include the following:
Diabetes mellitus
Pregnancy
Alcoholism
Hepatic failure
Renal failure
Corticosteroid treatment
In 43% of adult cases, however, no underlying disease is present.
Meningococcal meningitis
N meningitidis is a gram-negative diplococcus that is carried in the nasopharynx of
otherwise healthy individuals. It initiates invasion by penetrating the airway epithelial
surface. The precise mechanism by which this occurs is unclear, but recent viral or
mycoplasmal infection has been reported to disrupt the epithelial surface and
facilitate invasion by meningococcus.
Most sporadic cases of meningococcal meningitis (95-97%) are caused by
serogroups B, C, and Y, whereas the A and C strains are observed in epidemics (<
3% of cases). Currently, N meningitidis is the leading cause of bacterial meningitis in
children and young adults, accounting for 59% of cases.
Risk factors for meningococcal meningitis include the following:
Deficiencies in terminal complement components (eg, membrane attack complex,
C5-C9), which increases attack rates but is associated with surprisingly lower
mortality rates
Properdin defects that increase the risk of invasive disease
Antecedent viral infection, chronic medical illness, corticosteroid use, and active or
passive smoking
Crowded living conditions, as is observed in college dormitories (college freshmen
living in dormitories are at highest risk) and military facilities, which has been
reported in clustering of cases
Listeria monocytogenes meningitis
Listeria monocytogenes is a small gram-positive bacillus that causes 3% of bacterial
meningitis cases and is associated with one of the highest mortalities (20%).
[5]
The
organism is widespread in nature and has been isolated in the stool of 5% of healthy
adults. Most human cases appear to be food-borne.
L monocytogenes is a common food contaminant, with a recovery rate of up to 70%
from raw meat, vegetables, and meats. Outbreaks have been associated with
consumption of contaminated coleslaw, milk, cheese, and alfalfa tablets.
Groups at risk include the following:
Pregnant women
Infants and children
Elderly individuals (>60 years)
Patients with alcoholism
Adults who are immunosuppressed (eg, steroid users, transplant recipients, or
persons with AIDS)
Individuals with chronic liver and renal disease
Individuals with diabetes
Persons with iron-overload conditions (eg, hemochromatosis or transfusion-
induced iron overload)
Meningitis caused by gram-negative bacilli
Aerobic gram-negative bacilli include the following:
Escherichia coli
Klebsiella pneumoniae
Serratia marcescens
P aeruginosa
Salmonella species
Gram-negative bacilli can cause meningitis in certain groups of patients. E coli is a
common agent of meningitis among neonates. Other predisposing risk factors for
meningitis associated with gram-negative bacilli include the following:
Neurosurgical procedures or intracranial manipulation
Old age
Immunosuppression
High-grade gram-negative bacillary bacteremia
Disseminated strongyloidiasis
Disseminated strongyloidiasis has been reported as a classic cause of gram-
negative bacillary bacteremia, as a result of the translocation of gut microflora with
the Strongyloides stercoralis larvae during hyperinfection syndrome.
Staphylococcal meningitis
Staphylococci are gram-positive cocci that are part of the normal skin flora.
Meningitis caused by staphylococci is associated with the following risk factors:
Neurosurgery
Head trauma
Presence of CSF shunts
Infective endocarditis and paraspinal infection
S epidermidis is the most common cause of meningitis in patients with CNS (ie,
ventriculoperitoneal) shunts. (See Staphylococcal Meningitis.)
Aseptic meningitis
Aseptic meningitis is one of the most common infections of the meninges. If
appropriate diagnostic methods are employed, a specific viral etiology is identified in
50-60% of cases of aseptic meningitis. However, aseptic meningitis can also be
caused by bacteria, fungi, and parasites (see Table 1 below). It is noteworthy that
partially treated bacterial meningitis accounts for a large number of meningitis cases
with a negative microbiologic workup.
Table 1. Infectious Agents Causing Aseptic Meningitis (Open Table in a new
window)
Category Agent
Bacteria Partially treated bacterial meningitis
Listeria monocytogenes
Brucella spp
Rickettsia rickettsii
Ehrlichia spp
Mycoplasma pneumoniae
Borrelia burgdorferi
Treponema pallidum
Leptospira spp
Mycobacterium tuberculosis
Nocardia spp
Parasites Naegleria fowleri
Acanthamoeba spp
Balamuthia spp
Angiostrongylus cantonensis
Gnathostoma spinigerum
Baylisascaris procyonis
Strongyloides stercoralis
Taenia solium (cysticercosis)
Fungi Cryptococcus neoformans
Coccidioides immitis
Blastomyces dermatitidis
Histoplasma capsulatum
Candida spp
Aspergillus spp
Viruses Enterovirus Poliovirus
Echovirus
Coxsackievirus A
Coxsackievirus B
Enterovirus 68-71
Herpesvirus (HSV) HSV-1 and HSV-2
Varicella-zoster virus
Epstein-Barr virus
Cytomegalovirus
HHV-6 and HHV-7
Paramyxovirus Mumps virus
Measles virus
Togavirus Rubella virus
Flavivirus West Nile virus
Japanese encephalitis virus
St Louis encephalitis virus
Bunyavirus California encephalitis virus
La Crosse encephalitis virus
Alphavirus Eastern equine encephalitis virus
Western equine encephalitis virus
Venezuelan encephalitis virus
Reovirus Colorado tick fever virus
Arenavirus LCM virus
Rhabdovirus Rabies virus
Retrovirus HIV
HHV = human herpesvirus; HSV = herpes simplex virus; LCM = lymphocytic
choriomeningitis.
Enteroviruses account for of the majority of cases of aseptic meningitis in children,
but West Nile virus and HSV-2 account for a substantial proportion of cases in
adults. The enteroviruses belong to the family Picornaviridae and are further
classified as follows:
Poliovirus (3 serotypes)
Coxsackievirus A (23 serotypes)
Coxsackievirus B (6 serotypes)
Echovirus (31 serotypes)
Newly recognized enterovirus serotypes 68-71
Enteroviruses are usually spread by fecal-oral or respiratory routes. Infection occurs
during summer and fall in temperate climates and year-round in tropical regions.
The nonpolio enteroviruses (NPEVs) account for approximately 90% of cases of viral
meningitis in which a specific pathogen can be identified.
Echovirus 30 was reported as the cause of an epidemic in Japan in 1991. It was also
reported as the cause of 20% of cases of aseptic meningitis reported to the Centers
for Disease Control and Prevention (CDC) in 1991.
The Herpesviridae family consists of large, DNA-containing enveloped viruses. Eight
members are known to cause human infections, and all have been implicated in
meningitis syndromes, with the exception of HHV-8 or Kaposi sarcomaassociated
virus.
HSV accounts for 0.5-3% of cases of aseptic meningitis; it is most commonly
associated with primary genital infection and is less likely during recurrences. HSV-1
is a cause of encephalitis, while HSV-2 more commonly causes meningitis. Although
Mollaret syndrome (a recurrent, but benign, aseptic meningitis syndrome) is more
frequently associated with HSV-2, HSV-1 has also been implicated as a cause.
Epstein-Barr virus (EBV, or HHV-4) and cytomegalovirus (CMV, or HHV-5) infection
may manifest as meningitis in patients with the mononucleosis syndrome. Varicella-
zoster virus (VZV, or HHV-3) and CMV cause meningitis in immunocompromised
hosts, especially patients with AIDS and transplant recipients. HHV-6 and HHV-7
have been reported to cause meningitis in transplant recipients.
The most common arthropod-borne viruses are West Nile virus, St Louis encephalitis
virus (a flavivirus), Colorado tick fever virus, and California encephalitis virus
(bunyavirus group, including La Crosse encephalitis virus). St Louis encephalitis
virus is a mosquito-borne flavivirus that may cause a febrile syndrome, aseptic
meningitis syndrome, and encephalitis. Other members of the flavivirus group that
may cause aseptic meningitis include tick-borne encephalitis virus and Japanese
encephalitis virus.
California encephalitis is a common childhood disease of the CNS that is caused by
a virus in the genus Bunyavirus. Most of the cases of California encephalitis are
probably caused by mosquito-borne La Crosse encephalitis virus.
LCM virus is a member of the arenaviruses, a family of single-stranded, RNA-
containing viruses in which rodents are the animal reservoir. The modes of
transmission include aerosols and direct contact with rodents. Outbreaks have also
been traced to infected laboratory mice and hamsters.
The mumps virus is the most common cause of aseptic meningitis in unimmunized
populations, occurring in 30% of all patients with mumps. Upon exposure, an
incubation period of approximately 5-10 days ensues, followed by a nonspecific
febrile illness and an acute onset of aseptic meningitis. This may be associated with
orchitis, arthritis, myocarditis, and alopecia.
Patients with acute HIV infection may present with aseptic meningitis syndrome,
usually as part of the mononucleosislike acute seroconversion phenomenon. HIV
should always be suspected as a cause of aseptic meningitis in a patient with risk
factors such as IV drug use or high-risk sexual behaviors. These patients will have
negative results on HIV serologic tests (eg, enzyme-linked immunosorbent assay
[ELISA] and Western blot); the diagnosis is made by the detection of serum HIV
RNA on polymerase chain reaction (PCR) testing or of HIV p24 antigen.
Adenovirus (serotypes 1, 6, 7, and 12) has been associated with cases of
meningoencephalitis. Chronic meningoencephalitis has been reported with serotypes
7, 12, and 32. The infection is usually acquired through a respiratory route.
Toscana virus meningitis or encephalitis should be considered in travelers returning
from the a Mediterranean country (eg, Italy, Spain, or Greece) during the summer.
Toscana viruses are transmitted by the bite of a sandfly. Toscana virus infection can
be diagnosed by performing paired serologies and CSF PCR, which in the United
States is available only through the CDC.
[6]
Chronic meningitis
Chronic meningitis can be caused by a wide range of infectious and noninfectious
etiologies (see Table 2 below).
Table 2. Causes of Chronic Meningitis (Open Table in a new window)
Category Agent
Bacteria Mycobacterium tuberculosis
Borrelia burgdorferi
Treponema pallidum
Brucella spp
Francisella tularensis
Nocardia spp
Actinomyces spp
Fungi Cryptococcus neoformans
Coccidioides immitis
Blastomyces dermatitidis
Histoplasma capsulatum
Candida albicans
Aspergillus spp
Sporothrix schenckii
Parasites Acanthamoeba spp
Naegleria fowleri
Angiostrongylus cantonensis
Gnathostoma spinigerum
Baylisascarisprocyonis
Schistosoma spp
Strongyloides stercoralis
Echinococcus granulosus
Brucellae are small gram-negative coccobacilli that cause zoonoses as a result of
infection with Brucella abortus, Brucella melitensis, Brucella suis, or Brucella
canis. Transmission to humans occurs after direct or indirect exposure to infected
animals (eg, sheep, goats, or cattle). Direct infection of the CNS occurs in fewer than
5% of cases, with most patients presenting with acute or chronic meningitis.
Persons at risk for brucellosis include individuals who had contact with infected
animals or their products (eg, through intake of unpasteurized milk products).
Veterinarians, abattoir workers, and laboratory workers dealing with these animals
are also at risk.
M tuberculosis is an acid-fast bacillus that causes a broad range of clinical illnesses
that can affect virtually any organ of the body. It is spread through airborne droplet
nuclei, and it infects one third of the worlds population. Involvement of the CNS with
tuberculous meningitis is usually caused by rupture of a tubercle into the
subarachnoid space.
Tuberculous meningitis should always be considered in the differential diagnosis of
patients with aseptic meningitis or chronic meningitis syndromes, especially those
with basilar meningitis, symptoms of more than 5 days duration, or cranial nerve
palsies. If tuberculous meningitis is suspected, antituberculosis therapy, with or
without steroids, should be empirically started.
Treponema pallidum is a slender, tightly coiled spirochete that is usually acquired by
sexual contact. Other modes of transmission include direct contact with an active
lesion, passage through the placenta, and blood transfusion (rare).
Borrelia burgdorferi, a tick-borne spirochete, is the agent of Lyme disease, the most
common vector-borne disease in the United States. Meningitis may be part of a triad
of neurologic manifestations of Lyme disease that also includes cranial neuritis and
radiculoneuritis. Lyme disease meningitis is typically associated with a facial palsy
that can sometimes be bilateral.
Cryptococcus neoformans is an encapsulated, yeastlike fungus that is ubiquitous. It
has been found in high concentrations in aged pigeon droppings and pigeon nesting
places. The 4 serotypes are designated A through D, with the A serotype causing
most human infections. Onset of cryptococcal meningitis may be acute, especially
among patients with AIDS.
Numerous cases occur in healthy hosts (eg, persons with no known T-cell defect);
however, approximately 50-80% of cases occur in immunocompromised hosts. At
particular risk are individuals with defects of T-cellmediated immunity, such as
persons with AIDS, organ transplant recipients, and other patients who use steroids,
cyclosporine, and other immunosuppressants. Cryptococcal meningitis has also
been reported in patients with idiopathic CD-4 lymphopenia, Hodgkin disease,
sarcoidosis, and cirrhosis.
Coccidioides immitis is a soil-based, dimorphic fungus that exists in mycelial and
yeast (spherule) forms. Persons at risk for coccidioidal meningitis include individuals
exposed to the endemic regions (eg, tourists and local populations) and those with
immune deficiency (eg, persons with AIDS and organ transplant recipients).
Blastomyces dermatitidis is a dimorphic fungus that has been reported to be
endemic in North America (eg, in the Mississippi and Ohio River basins). It has also
been isolated from parts of Central America, South America, the Middle East, and
India. Its natural habitat is not well defined. Soil that is rich in decaying matter and
environments around riverbanks and waterways have been demonstrated to
harbor B dermatitidis during outbreaks and are thought to be risk factors for
acquiring the infection.
Inhalation of the conidia establishes a pulmonary infection. Dissemination may occur
in certain individuals, including those with underlying immune deficiency (eg, from
HIV or pharmaceutical agents) and extremes of age, and may involve the skin,
bones and joints, genitourinary tract, and CNS. Involvement of the CNS occurs in
fewer than 5% of cases.
Histoplasma capsulatum is one of the dimorphic fungi that exist in mycelial and yeast
forms. It is usually found in soil and can occasionally cause a chronic meningitis. The
preferred means of making the diagnosis is CSF histoplasma antigen detection.
Candida species are ubiquitous in nature. They are normal commensals in humans
and are found in the skin, the GI tract, and the female genital tract. The most
common species is Candida albicans, but the incidence of non-albicanscandidal
infections (eg, Candida tropicalis) is increasing, including species with antifungal
resistance (eg, Candida krusei and Candida glabrata).
Involvement of the CNS usually follows hematogenous dissemination. The most
important predisposing risks for acquiring disseminated candidal infection appear to
be iatrogenic (eg, the administration of broad-spectrum antibiotics and the use of
indwelling devices such as urinary and vascular catheters). Prematurity in neonates
is considered a predisposing risk factor as well. Infection may also follow
neurosurgical procedures, such as placement of ventricular shunts.
Sporothrix schenckii is an endemic dimorphic fungus that is often isolated from soil,
plants, and plant products. Human infections are characteristically lymphocutaneous.
Extracutaneous manifestations of sporotrichosis may occur, though meningeal
sporotrichosis, which is the most severe form, is a rare complication. AIDS is a
reported underlying risk factor in many described cases and is associated with a
poor outcome.
Infection with free-living amebas is an infrequent but often life-threatening human
illness, even in immunocompetent individuals. N fowleri is the only species
ofNaegleria recognized to be pathogenic in humans, and it is the agent of primary
amebic meningoencephalitis (PAM). The parasite has been isolated in lakes, pools,
ponds, rivers, tap water, and soil.
Infection occurs when a person is swimming or playing in contaminated water
sources (eg, inadequately chlorinated water and sources associated with poor
decontamination techniques). The N fowleri amebas invade the CNS through the
nasal mucosa and cribriform plate.
PAM occurs in 2 forms. The first is characterized by an acute onset of high fever,
photophobia, headache, and altered mental status, similar to bacterial meningitis,
occurring within 1 week after exposure. Because it is acquired via the nasal area,
olfactory nerve involvement may manifest as abnormal smell sensation. Death
occurs in 3 days in patients who are not treated. The second form, the subacute or
chronic form, consists of an insidious onset of low-grade fever, headache, and focal
neurologic signs. Duration of illness is weeks to few months.
Acanthamoeba and Balamuthia cause granulomatous amebic encephalitis, which is
a subacute opportunistic infection that spreads hematogenously from the primary
site of infection (skin or lungs) to the CNS and causes an encephalitis syndrome.
These cases can be difficult to distinguish from culture-negative meningitis.
Angiostrongylus cantonensis, the rat lungworm, can cause eosinophilic meningitis
(pleocytosis with more than 10% eosinophils) in humans. The adult parasite resides
in the lungs of rats. Its eggs hatch, and the larval stages are expelled in the feces.
The larvae develop in the intermediate host, usually land snails, freshwater prawns,
and crabs. Humans acquire the infection by ingesting raw mollusks.
Gnathostoma spinigerum, a GI parasite of wild and domestic dogs and cats, may
cause eosinophilic meningoencephalitis. Humans acquire the infection after
ingesting undercooked infected fish and poultry.
Baylisascaris procyonis is an ascarid parasite that is prevalent in the raccoon
populations in the United States and rarely causes human eosinophilic
meningoencephalitis. Human infections occur after accidental ingestion of food
products contaminated with raccoon feces.
Additional causes of meningitis
Congenital malformation of the stapedial footplate has been implicated in the
development of meningitis. Head and neck surgery, penetrating head injury,
comminuted skull fracture, and osteomyelitic erosion may infrequently result in direct
implantation of bacteria into the meninges. Skull fractures can tear the dura and
cause a CSF fistula, especially in the region of the frontal ethmoid sinuses. Patients
with any of these conditions are at risk for bacterial meningitis.
Epidemiology
The incidence of meningitis varies according to the specific etiologic agent, as well
as in conjunction with a nations medical resources. The incidence is presumed to be
higher in developing countries because of less access to preventive services, such
as vaccination. In these countries, the incidence has been reported to be 10 times
higher than that in developed countries.
Meningitis affects people of all races. In the United States, black people have a
higher reported rate of meningitis than white people and Hispanic people.
Epidemiology of bacterial meningitis
With almost 4100 cases and 500 deaths occurring annually in the United States,
bacterial meningitis continues to be a significant source of morbidity and mortality.
The annual incidence in the United States is 1.33 cases per 100,000 population.
[5]
Meningococcal meningitis is endemic in parts of Africa, India, and other developing
areas. Periodic epidemics occur in the so-called sub-Saharan meningitis belt, as
well as among religious pilgrims traveling to Saudi Arabia for the Hajj. In parts of
Africa, widespread epidemics of meningococcal meningitis occur regularly. In 1996,
the biggest wave of meningococcal meningitis outbreaks ever recorded arose in
West Africa. An estimated 250,000 cases and 25,000 deaths occurred in Niger,
Nigeria, Burkina Faso, Chad, and Mali.
The incidence of neonatal bacterial meningitis is 0.25-1 case per 1000 live births. In
addition, the incidence is 0.15 case per 1000 full-term births and 2.5 cases per 1000
premature births. Approximately 30% of newborns with clinical sepsis have
associated bacterial meningitis.
N meningitidis causes approximately 4 cases per 100,000 children aged 1-23
months. The risk of secondary meningitis is 1% for family contacts and 0.1% for
daycare contacts. The rate of meningitis caused by S pneumoniae is 6.5 cases per
100,000 children aged 1-23 months.
Previously, Hib, N meningitidis, and S pneumoniae accounted for more than 80% of
cases of bacterial meningitis. Since the late 20th century, however, the epidemiology
of bacterial meningitis has been substantially changed by multiple developments.
The overall incidence of bacterial meningitis in the US declined from 2.0 to 1.38
cases per 100,000 population between 1998 and 2007.
[5]
This was partially because
of the widespread use of the Hib vaccination, which decreased the incidence of H
influenzae meningitis by more than 90% (see Table 3 below). Routine Hib
vaccination has nearly eliminating this pathogen as a cause of meningitis in many
developed countries.
More recent prevention measures such as the pneumococcal conjugate vaccine and
universal screening of pregnant women for GBS have further changed the
epidemiology of bacterial meningitis.
Table 3. Changing Epidemiology of Acute Bacterial Meningitis in United
States*(Open Table in a new window)
Bacteria 1978-1981 1986 1995 1998-
2007
Haemophilus influenzae 48% 45% 7% 6.7%
Listeria monocytogenes 2% 3% 8% 3.4%
Neisseria meningitidis 20% 14% 25% 13.9%
Streptococcus agalactiae (group B streptococcus) 3% 6% 12% 18.1%
Streptococcus pneumoniae 13% 18% 47% 58%
*Nosocomial meningitis is not included; these data include only the 5 major
meningeal pathogens.
The number of cases of invasive H influenzae disease among children younger than
5 years that were reported to the CDC declined from 20,000 in 1987 to 255 in 1998.
This shift has reportedly been less dramatic in developing countries, where the use
of Hib vaccine is not as widespread.
Because the frequency of bacterial meningitis in children has declined, the condition
is becoming more of a disease of adults. Whereas the median age for persons with
bacterial meningitis was 25 years in 1998, it was 15 months in 1986.
[7]
The introduction of vaccines against S pneumoniae has substantially reduced the
incidence of pneumococcal meningitis in children. Routine screening for GBS in
pregnant women may have also reduced the incidence of meningitis from this
pathogen . Routine vaccination against serogroup C meningococcus may also
reduce the incidence of N meningitidis infections. During a 1998-2007 survey, the
incidence of meningitis declined by 31%,
[5]
a decrease that can be credited to
vaccination programs.
Newborns are at highest risk for acute bacterial meningitis. After the first month of
life, the peak incidence is in infants aged 3-8 months. In addition, the incidence is
increased in persons aged 60 years and older, independent of other factors. The
annual incidence ranges from 1.7 to 7.2 cases per 100,000 adults; the mean annual
incidence has been reported as 3.8 cases per 100,000 adults. Of patients with
bacterial meningitis, 61% had no previous or present accompanying diseases that
may have predisposed them to meningitis.
Depending on their age, individuals are also predisposed to meningitis from other
etiologic agents (see Table 4 below). E coli K1 meningitis and S agalactiaemeningitis
are common among neonates, and L monocytogenes meningitis is common among
neonates and the elderly. (The development of neonatal meningitis is related to labor
and delivery; it results from colonized pathogens in the maternal intestinal or genital
tract, immaturity, and environment.)
Table 4. Most Common Bacterial Pathogens on Basis of Age and Predisposing
Risks (Open Table in a new window)
Risk or Predisposing Factor Bacterial Pathogen
Age 0-4 weeks Streptococcus agalactiae (GBS)
Escherichia coli K1
Listeria monocytogenes
Age 4-12 weeks S agalactiae
E coli
Haemophilus influenzae
Streptococcus pneumoniae
Neisseria meningitidis
Age 3 months to 18 years N meningitidis
S pneumoniae
H influenzae
Age 18-50 years S pneumoniae
N meningitidis
H influenzae
Age >50 years S pneumoniae
N meningitidis
L monocytogenes
Aerobic gram-negative bacilli
Immunocompromised state S pneumoniae
N meningitidis
L monocytogenes
Aerobic gram-negative bacilli
Intracranial manipulation, including
neurosurgery
Staphylococcus aureus
Coagulase-negative staphylococci
Aerobic gram-negative bacilli,
including Pseudomonas aeruginosa
Basilar skull fracture S pneumoniae
H influenzae
Group A streptococci
CSF shunts Coagulase-negative staphylococci
S aureus
Aerobic gram-negative bacilli
Propionibacterium acnes
CSF = cerebrospinal fluid; GBS = group B streptococcus.
The reported attack rate for bacterial meningitis is 3.3 male cases per 100,000
population, compared with 2.6 female cases per 100,000 population. However, in
meningitis caused by the mumps virus, males and females are affected equally. In
neonates, the male-to-female ratio is 3:1.
Epidemiology of specific bacterial pathogens of acute meningitis
H influenzae meningitis primarily affects infants younger than 2 years. S
agalactiae meningitis occurs principally during the first 12 weeks of life but has also
been reported in adults, primarily affecting individuals older than age 60 years. The
overall case-fatality rate in adults is 34%. Among the bacterial agents that cause
meningitis, S pneumoniae is associated with one of the highest mortalities (19-26%).
Epidemiology of aseptic meningitis
Aseptic meningitis has a reported incidence of 10.9 cases per 100,000 person-years.
It occurs in individuals of all ages but is more common in children, especially during
summer. No racial differences are reported. Aseptic meningitis tends to occur 3
times more frequently in males than in females.
Viruses are the major cause of aseptic meningitis. The enteroviruses are distributed
worldwide, and the infection rates vary according to the season of the year and a
populations age and socioeconomic status. Most enteroviral infections occur in
individuals who are younger than 15 years, with the highest attack rates in children
who are younger than 1 year.
Arboviruses are an important cause of aseptic meningitis and encephalitis in the
summer and fall months in the United States. West Nile virus was introduced to the
United States in 1999 and has now spread throughout the continent. In 2012, the
largest outbreak of West Nile virus infection to date occurred in the United States,
with 5387 cases reported (about half of which were neuroinvasive disease, such as
meningitis or encephalitis) and a 4.5% mortality.
[8]
West Nile virus can also cause
acute flaccid paralysis, retinitis and nephropathy.
Other less common arboviruses include St Louis encephalitis virus, Jamestown
canyon virus, La Crosse encephalitis virus, Powassan encephalitis virus, and
Eastern equine encephalitis virus. In the United States, the last epidemic of St Louis
encephalitis was in Monroe, Louisiana, in 2001; 63 cases were reported, with 3
deaths (4.7% mortality). Infection with the La Crosse encephalitis virus also usually
occurs during the summer and early fall, with symptoms again being typical of acute
aseptic meningitis.
[9]
Infections with the LCM virus occur worldwide. Most human cases occur among
young adults during autumn.
Of fungal causes, B dermatitidis is reportedly endemic in North America (eg,
Mississippi and Ohio River basins). It has also been isolated from parts of Central
America, South America, the Middle East, and India. H capsulatum has been
reported from many areas of the world, with the Mississippi and Ohio River valleys
being the most endemic regions in North America.
Of parasitic causes, A cantonensis is common in Southeast Asia and the Pacific
Islands. It has also been found in rats outside this region, particularly in regions of
Africa, Puerto Rico, and Louisiana, presumably introduced by ship-borne rats from
endemic areas. G spinigerum is common in Southeast Asia, China, and Japan but
has been reported sporadically worldwide.
Epidemiology of chronic meningitis
Brucella -associated chronic meningitis has a worldwide distribution and is common
in the Middle East, India, Mexico, and Central and South America. In the United
States, after the control of bovine infections, the incidence decreased to less than
0.5 cases per 100,000 population, and only 79 cases were reported to the CDC in
1998.
M tuberculosis is worldwide in distribution, and humans are its only reservoir. In
1997, the estimated case rates among endemic countries ranged from 62 to 411
cases per 100,000 population.
B burgdorferi is a tick-borne spirochete that is found in the temperate regions of
much of the northern hemisphere. Endemic regions include North America (eg, the
northeastern United States, Minnesota, Wisconsin, and parts of California and
Oregon), Europe, and Asia.
C neoformans has a worldwide distribution. Serotypes B and C have been restricted
mostly to tropical and subtropical regions, and serotype B has been isolated from
eucalyptus trees.
The distribution of C immitis is limited to the endemic regions of the Western
Hemisphere, within the north and south 40 latitudes (ie, parts of the southwestern
United States, Mexico, and Central and South America). Persons who have migrated
from or traveled to endemic areas may experience onset of disease in other parts of
the world.
S schenckii has been reported worldwide. However, most cases come from the
tropical regions of the Americas.
Prognosis
Patients with meningitis who present with an impaired level of consciousness are at
increased risk for neurologic sequelae or death. A seizure during an episode of
meningitis also is a risk factor for mortality or neurologic sequelae, particularly if the
seizure is prolonged or difficult to control.
In bacterial meningitis, several risk factors are associated with death and with
neurologic disability. A risk score has been derived and validated in adults with
bacterial meningitis. This score includes the following variables, which are
associated with an adverse clinical outcome
[10]
:
Older age
Increased heart rate
Lower Glasgow Coma Scale score
Cranial nerve palsies
CSF leukocyte count lower than 1000/L
Gram-positive cocci on CSF Gram stain
Advanced bacterial meningitis can lead to brain damage, coma, and death. In 50%
of patients, several complications may develop in the days to weeks following
infection. Long-term sequelae are seen in as many as 30% of survivors and vary
with etiologic agent, patient age, presenting features, and hospital course. Patients
usually have subtle CNS changes.
Serious complications include the following:
Hearing loss
Cortical blindness
Other cranial nerve dysfunction
Paralysis
Muscular hypertonia
Ataxia
Multiple seizures
Mental motor retardation
Focal paralysis
Ataxia
Subdural effusions
Hydrocephalus
Cerebral atrophy
Risk factors for hearing loss after pneumococcal meningitis are female gender, older
age, severe meningitis, and infection with certain pneumococcal serotypes (eg,
12F).
[11]
Delayed complications include the following:
Decreased hearing or deafness
Other cranial nerve dysfunctions
Multiple seizures
Focal paralysis
Subdural effusions
Hydrocephalus
Intellectual deficits
Ataxia
Blindness
Waterhouse-Friderichsen syndrome
Peripheral gangrene
Seizures are a common and important complication, occurring in approximately one
fifth of patients. The incidence is higher in patients younger than 1 year, reaching
40%. Approximately one half of patients with this complication have repeated
seizures. Patients may die as a result of diffuse CNS ischemic injury or systemic
complications.
Even with effective antimicrobial therapy, significant neurologic complications have
been reported to occur in as many as 30% of survivors of bacterial meningitis. Close
monitoring for the development of these complications is essential.
Mortality for bacterial meningitis is highest in the first year of life, decreases in
midlife, and increases again in old age. Bacterial meningitis is fatal in 1 in 10 cases,
and 1 of every 7 survivors is left with a severe handicap, such as deafness or brain
injury.
The prognosis in patients with meningitis caused by opportunistic pathogens
depends on the underlying immune function of the host. Many patients who survive
the disease require lifelong suppressive therapy (eg, long-term fluconazole for
suppression in patients with HIV-associated cryptococcal meningitis).
Among bacterial pathogens, S pneumoniae causes the highest mortality (20-30% in
adults, 10% in children) and morbidity (15%) in meningitis. If severe neurologic
impairment is evident at the time of presentation (or if the onset of illness is
extremely rapid), mortality is 50-90% and morbidity is even higher, even with
immediate medical treatment. Meningitis caused by L monocytogenes or gram-
negative bacilli also has a higher case-fatality rate than meningitis caused by other
bacterial agents.
Reported overall mortality for meningitis from specific bacterial organisms is as
follows:
S pneumoniae - 19-26%
H influenzae - 3-6%
N meningitidis - 3-13%
L monocytogenes - 15-29%
Patients with meningococcal meningitis have a better prognosis than do those with
pneumococcal meningitis, with a mortality of 4-5%; however, patients with
meningococcemia have a poor prognosis, with a mortality of 20-30%.
The mortality for viral meningitis without encephalitis is less than 1%. In patients with
deficient humoral immunity (eg, agammaglobulinemia), enteroviral meningitis may
have a fatal outcome. Patients with viral meningitis usually have a good prognosis
for recovery. The prognosis is worse for patients at the extremes of age (ie, < 2 or
>60 years) and those with significant comorbidities and underlying
immunodeficiency.
Patient Education
Patients and parents of young children should be educated about the benefits of
vaccination in preventing meningitis. Vaccination against N meningitidis is
recommended for all US college students.
Close contacts of patients with known or suspected N meningitidis or Hib meningitis
may require education regarding the need for prophylaxis. All contacts should be
instructed to come to the emergency department immediately at the first sign of
fever, sore throat, rash, or symptoms of meningitis. Rifampin prophylaxis only
eradicates the organism from the nasopharynx; it is ineffective against invasive
disease.
For patient education information, see the Brain and Nervous System Center and
the Childrens Health Center, as well as Meningitis in Adults, Meningitis in
Children, Brain Infection, and Spinal Tap.
También podría gustarte
- Meningitis MedscapeDocumento73 páginasMeningitis MedscapeBujangAún no hay calificaciones
- dMAC Digest Volume 5 No 2: MeningitisDe EveranddMAC Digest Volume 5 No 2: MeningitisCalificación: 5 de 5 estrellas5/5 (3)
- Healthcare Professionals Guide to MeningitisDocumento26 páginasHealthcare Professionals Guide to MeningitisdilaAún no hay calificaciones
- Definition-: Neisseria Meningitidis Invading The Subarachnoid Space of The BrainDocumento12 páginasDefinition-: Neisseria Meningitidis Invading The Subarachnoid Space of The BrainAmit MartinAún no hay calificaciones
- Meningitis &encephalitisDocumento9 páginasMeningitis &encephalitisSnIP StandredAún no hay calificaciones
- Meningitis and Dementia: Dr. Lubna DwerijDocumento52 páginasMeningitis and Dementia: Dr. Lubna DwerijNoor MajaliAún no hay calificaciones
- Meningitis: Author InformationDocumento10 páginasMeningitis: Author Informationakbar011512Aún no hay calificaciones
- Bacterial Meningitis: Etiology and PathophysiologyDocumento9 páginasBacterial Meningitis: Etiology and PathophysiologyIndra Dwi VerawatiAún no hay calificaciones
- Therapy LV CNS 4th Year KirubelDocumento156 páginasTherapy LV CNS 4th Year Kirubelpblinder1319Aún no hay calificaciones
- Recurrent MeningitisDocumento14 páginasRecurrent Meningitisidno1008Aún no hay calificaciones
- Neurological Infections: Gerard Gabriel P. Reotutar, RM, RN, MANDocumento33 páginasNeurological Infections: Gerard Gabriel P. Reotutar, RM, RN, MANJeremiash Noblesala Dela CruzAún no hay calificaciones
- Meningitis Clinical PresentationDocumento10 páginasMeningitis Clinical PresentationAniwat NillakarnAún no hay calificaciones
- TB MeningitisDocumento18 páginasTB Meningitisjesa34Aún no hay calificaciones
- Intracranial Infection - Prof SunartiniDocumento12 páginasIntracranial Infection - Prof SunartiniFranciscus BuwanaAún no hay calificaciones
- Acute Bacterial MeningitisDocumento15 páginasAcute Bacterial MeningitisOana StefanAún no hay calificaciones
- Meningitis - StatPearls - NCBI BookshelfDocumento13 páginasMeningitis - StatPearls - NCBI Bookshelfaslan tonapaAún no hay calificaciones
- International Journal of Pharmaceutical Science Invention (IJPSI)Documento4 páginasInternational Journal of Pharmaceutical Science Invention (IJPSI)inventionjournalsAún no hay calificaciones
- Infectious Diseases - 03Documento23 páginasInfectious Diseases - 03Arthur YanezAún no hay calificaciones
- Meningitis and Encephalitis: Causes, Symptoms and TreatmentsDocumento71 páginasMeningitis and Encephalitis: Causes, Symptoms and TreatmentsKaif KhanAún no hay calificaciones
- Menigitis EncephalitisDocumento63 páginasMenigitis EncephalitisHussain AzharAún no hay calificaciones
- Acute Isolated MyocarditisDocumento20 páginasAcute Isolated Myocarditismerin sunilAún no hay calificaciones
- Meningitis: Classification and External ResourcesDocumento8 páginasMeningitis: Classification and External ResourcesChoco MuchoAún no hay calificaciones
- MYOCARDITIS: A GUIDE TO THE INFLAMMATORY HEART DISEASEDocumento56 páginasMYOCARDITIS: A GUIDE TO THE INFLAMMATORY HEART DISEASENANNY MARSIDINAún no hay calificaciones
- Blok-22-Meningitis-Tuberkulosis Fakhrurrozi PratamaDocumento16 páginasBlok-22-Meningitis-Tuberkulosis Fakhrurrozi PratamaFakhrurrozi PratamaAún no hay calificaciones
- Neurological InfectionsDocumento21 páginasNeurological Infectionsfortuneholiness11Aún no hay calificaciones
- EncephalitisDocumento37 páginasEncephalitisPRADEEPAún no hay calificaciones
- CPPP PP P PP PPPPPPP PPDocumento3 páginasCPPP PP P PP PPPPPPP PPangelieballesterosAún no hay calificaciones
- MyocarditisDocumento29 páginasMyocarditispanvilai0% (1)
- Case Study Bacterial MeningitisDocumento5 páginasCase Study Bacterial MeningitisChristine SaliganAún no hay calificaciones
- Viral MyocarditisDocumento42 páginasViral MyocarditisAlishba AtifAún no hay calificaciones
- Meningitis Up To DateDocumento27 páginasMeningitis Up To DateJonathan Gustavo MenaAún no hay calificaciones
- Meningitis 1Documento23 páginasMeningitis 1Juan RodriguezAún no hay calificaciones
- Biomarkers For Myocarditis and Inflammatory CardiomyopathyDocumento10 páginasBiomarkers For Myocarditis and Inflammatory CardiomyopathyCarlos Peña PaterninaAún no hay calificaciones
- TB MeningitisDocumento10 páginasTB MeningitisEveline YuniartiAún no hay calificaciones
- Pathology of CNS Infections: Principal Routes, Meningitis Types, Focal InfectionsDocumento39 páginasPathology of CNS Infections: Principal Routes, Meningitis Types, Focal Infectionsskin_docAún no hay calificaciones
- Presentation On MeningitisDocumento51 páginasPresentation On Meningitissushma shresthaAún no hay calificaciones
- Encephalitis: From Wikipedia, The Free EncyclopediaDocumento4 páginasEncephalitis: From Wikipedia, The Free Encyclopediamahesh2995Aún no hay calificaciones
- Tuberculous Meningitis Differential DiagnosesDocumento3 páginasTuberculous Meningitis Differential DiagnosesBenzi AbesAún no hay calificaciones
- Understanding Meningitis: Causes, Symptoms, Diagnosis and TreatmentDocumento41 páginasUnderstanding Meningitis: Causes, Symptoms, Diagnosis and TreatmentO'Mark AndrewsAún no hay calificaciones
- Sepsis and Septic Shock - Critical Care MedicineDocumento2 páginasSepsis and Septic Shock - Critical Care MedicineMihaela MoraruAún no hay calificaciones
- Case Study Bacterial MeningitisDocumento4 páginasCase Study Bacterial MeningitisChristine SaliganAún no hay calificaciones
- Approach To The Patient With Suspected Infection of SNCDocumento12 páginasApproach To The Patient With Suspected Infection of SNCJosé Miguel FerreiraAún no hay calificaciones
- Treatment: Tensive Care Unit Is Often NecessaryDocumento12 páginasTreatment: Tensive Care Unit Is Often NecessaryCocosul Cocosului CocosaruluiAún no hay calificaciones
- meningitisDocumento2 páginasmeningitiswydiake3Aún no hay calificaciones
- Pathology of The Central Nervous SystemDocumento78 páginasPathology of The Central Nervous Systemعلي عليAún no hay calificaciones
- The Cause of Death in Bacterial Meningitis: Researcharticle Open AccessDocumento9 páginasThe Cause of Death in Bacterial Meningitis: Researcharticle Open AccessAlexandra CardosoAún no hay calificaciones
- Neurological Infections: By, Mr. Anish Ghosh M.Sc. Nursing 2 YearDocumento57 páginasNeurological Infections: By, Mr. Anish Ghosh M.Sc. Nursing 2 YearAnish GhoshAún no hay calificaciones
- Blank 10Documento7 páginasBlank 10Leomer Calderon jr.Aún no hay calificaciones
- Understanding Meningitis: Causes, Symptoms and TreatmentDocumento49 páginasUnderstanding Meningitis: Causes, Symptoms and TreatmentDrAbhilash RMAún no hay calificaciones
- Managing Meningoencephalitis in Indian ICU: Neurocritical CareDocumento5 páginasManaging Meningoencephalitis in Indian ICU: Neurocritical CareerikafebriyanarAún no hay calificaciones
- Pubid-848078832 1010 PDFDocumento10 páginasPubid-848078832 1010 PDFCocosul Cocosului CocosaruluiAún no hay calificaciones
- BffsDocumento60 páginasBffsdrewantaAún no hay calificaciones
- Meningitis (Physical Exam)Documento6 páginasMeningitis (Physical Exam)MohammadAwitAún no hay calificaciones
- Chapter 22Documento7 páginasChapter 22Lyra Ortega OliquinoAún no hay calificaciones
- Microorganisms Related To Cardiac Infections: Ramlan SadeliDocumento39 páginasMicroorganisms Related To Cardiac Infections: Ramlan SadeliPriya GuptaAún no hay calificaciones
- B.I.Sharapov - Thefounderofthemoldavian Neurologicalschool: of PrimaryDocumento4 páginasB.I.Sharapov - Thefounderofthemoldavian Neurologicalschool: of PrimaryDoina CneagnitchiAún no hay calificaciones
- Pseudomonas Aeruginosa InfectionsDocumento22 páginasPseudomonas Aeruginosa InfectionsMiguel RomeroAún no hay calificaciones
- D17B PresentationDocumento28 páginasD17B PresentationBruno KandatamAún no hay calificaciones
- Meningitis: Meninges Brain Spinal CordDocumento12 páginasMeningitis: Meninges Brain Spinal CordAmanzeAún no hay calificaciones
- 7 Contoh Analytical TextDocumento10 páginas7 Contoh Analytical TextGaluh NurfadillahAún no hay calificaciones
- The Generic Structure of Narrative Adalah OrientationDocumento1 páginaThe Generic Structure of Narrative Adalah OrientationGaluh NurfadillahAún no hay calificaciones
- Dr. Maut (Spinal and Spinal Cord Trauma), Dr. Andry UsmanDocumento66 páginasDr. Maut (Spinal and Spinal Cord Trauma), Dr. Andry UsmanGaluh NurfadillahAún no hay calificaciones
- 15.cell Injury 1Documento8 páginas15.cell Injury 1Raissa Alfaathir HeriAún no hay calificaciones
- Diagnostico y Tratamiento de La Diabetes Tipo II ADA 2014Documento67 páginasDiagnostico y Tratamiento de La Diabetes Tipo II ADA 2014caluca1987Aún no hay calificaciones
- 5Documento20 páginas5Galuh NurfadillahAún no hay calificaciones
- Pain Management Guidelines 15-11-2012Documento62 páginasPain Management Guidelines 15-11-2012Galuh NurfadillahAún no hay calificaciones
- Signs and Symptoms: Clinical PresentationDocumento11 páginasSigns and Symptoms: Clinical PresentationGaluh NurfadillahAún no hay calificaciones
- Prognosis: MeningitisDocumento3 páginasPrognosis: MeningitisGaluh NurfadillahAún no hay calificaciones
- 2Documento34 páginas2Galuh NurfadillahAún no hay calificaciones
- Elizabeth Oneill Award 2014 Indonesian ApplicantsDocumento4 páginasElizabeth Oneill Award 2014 Indonesian ApplicantsGaluh NurfadillahAún no hay calificaciones
- Case ReportDocumento4 páginasCase ReportGaluh NurfadillahAún no hay calificaciones
- Tuberculous MeningitisDocumento11 páginasTuberculous MeningitiszuhriAún no hay calificaciones
- Infection and EpizootiologyDocumento3 páginasInfection and EpizootiologyAdrian Mausig100% (1)
- 2015 Antibiotic Prophylaxis DentalDocumento5 páginas2015 Antibiotic Prophylaxis DentalSyedMuhammadJunaidAún no hay calificaciones
- Staphylococci Chapter SummaryDocumento8 páginasStaphylococci Chapter SummarySamanthaAún no hay calificaciones
- Adam Low - CLABSI Best Practice and BundlesDocumento34 páginasAdam Low - CLABSI Best Practice and Bundlesvera indrawatiAún no hay calificaciones
- Sphingomonas Paucimobilis A Persistent Gram-Negati PDFDocumento6 páginasSphingomonas Paucimobilis A Persistent Gram-Negati PDFRita Mardhiya WAún no hay calificaciones
- Guías Infecciones Asociadas A Cateter IDSA 2009Documento45 páginasGuías Infecciones Asociadas A Cateter IDSA 2009pablocaballiniAún no hay calificaciones
- Case Files® EnterococcusDocumento4 páginasCase Files® EnterococcusAHMAD ADE SAPUTRAAún no hay calificaciones
- Clinical Practice Guideline For The Use of Antimicrobial Agents in Nutropenic Patients CID 11Documento38 páginasClinical Practice Guideline For The Use of Antimicrobial Agents in Nutropenic Patients CID 11Mariela ColomboAún no hay calificaciones
- New Jersey Health Department Report RE: Dr. John VecchioneDocumento11 páginasNew Jersey Health Department Report RE: Dr. John VecchioneNews12NJWebAún no hay calificaciones
- Blood CultureDocumento38 páginasBlood CultureGlucose DRglucoseAún no hay calificaciones
- Emergent Management of Pediatric Patients With FeverDocumento15 páginasEmergent Management of Pediatric Patients With FeverPandu WinataAún no hay calificaciones
- Bacteremia Fungemia, and Blood Cul Tures-V1Documento53 páginasBacteremia Fungemia, and Blood Cul Tures-V1Alok MishraAún no hay calificaciones
- Aklan State University Nursing Student Obstetrical AssessmentDocumento14 páginasAklan State University Nursing Student Obstetrical AssessmentKaren TangAún no hay calificaciones
- Bloodstream Infection MBDocumento47 páginasBloodstream Infection MBDarshan Koirala100% (1)
- Infection Control in ICU'sDocumento58 páginasInfection Control in ICU'stummalapalli venkateswara rao100% (1)
- Nag2019 PDFDocumento288 páginasNag2019 PDFmashupAún no hay calificaciones
- Review of Related Literature and Studies: Pediatr Dent 10 (4), 328B329, 2009members of The College ofDocumento4 páginasReview of Related Literature and Studies: Pediatr Dent 10 (4), 328B329, 2009members of The College ofZoella ZoeAún no hay calificaciones
- Clinical MicrobiologyDocumento91 páginasClinical MicrobiologyDr. Jayesh Patidar67% (3)
- Microbiology Infectious Disease Case StudiesDocumento18 páginasMicrobiology Infectious Disease Case StudiesKayeAún no hay calificaciones
- OMCDocumento37 páginasOMCyurie_ameliaAún no hay calificaciones
- Blood Culture BookletDocumento17 páginasBlood Culture BookletmikrougmAún no hay calificaciones
- Cochrane ReviewDocumento61 páginasCochrane ReviewponekAún no hay calificaciones
- Surveillance Manual 2012Documento122 páginasSurveillance Manual 2012abdullah100% (1)
- (Microbio) Staphyloccocus and Streptococcus-Dr. Salandanan (BHND)Documento16 páginas(Microbio) Staphyloccocus and Streptococcus-Dr. Salandanan (BHND)Lee Delos Santos100% (1)
- Infectious Diseases II PDFDocumento62 páginasInfectious Diseases II PDFhuong LAún no hay calificaciones
- Nursing Care Plan Neonatal SepsisDocumento2 páginasNursing Care Plan Neonatal Sepsisderic100% (20)
- Endoftalmitis EndogenaDocumento1 páginaEndoftalmitis EndogenaAlexander Fabián Rodríguez DíazAún no hay calificaciones
- Antibacterial Activity of Makabuhay Plant against Staphylococcus aureusDocumento34 páginasAntibacterial Activity of Makabuhay Plant against Staphylococcus aureusMayflor Baricaua100% (1)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionDe EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionCalificación: 4 de 5 estrellas4/5 (402)
- Why We Die: The New Science of Aging and the Quest for ImmortalityDe EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityCalificación: 3.5 de 5 estrellas3.5/5 (2)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisDe EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisCalificación: 4 de 5 estrellas4/5 (1)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedDe EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedCalificación: 5 de 5 estrellas5/5 (78)
- The Age of Magical Overthinking: Notes on Modern IrrationalityDe EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityCalificación: 4 de 5 estrellas4/5 (13)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeDe EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeAún no hay calificaciones
- The Comfort of Crows: A Backyard YearDe EverandThe Comfort of Crows: A Backyard YearCalificación: 4.5 de 5 estrellas4.5/5 (23)
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsDe EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsCalificación: 3.5 de 5 estrellas3.5/5 (3)
- Techniques Exercises And Tricks For Memory ImprovementDe EverandTechniques Exercises And Tricks For Memory ImprovementCalificación: 4.5 de 5 estrellas4.5/5 (40)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsDe EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsCalificación: 5 de 5 estrellas5/5 (1)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsDe EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsCalificación: 4.5 de 5 estrellas4.5/5 (169)
- The Obesity Code: Unlocking the Secrets of Weight LossDe EverandThe Obesity Code: Unlocking the Secrets of Weight LossCalificación: 5 de 5 estrellas5/5 (4)
- The Ultimate Guide To Memory Improvement TechniquesDe EverandThe Ultimate Guide To Memory Improvement TechniquesCalificación: 5 de 5 estrellas5/5 (34)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingDe EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingCalificación: 5 de 5 estrellas5/5 (4)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaDe EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaCalificación: 4.5 de 5 estrellas4.5/5 (266)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsDe EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsAún no hay calificaciones
- Summary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisDe EverandSummary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisCalificación: 5 de 5 estrellas5/5 (8)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingDe EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingCalificación: 3.5 de 5 estrellas3.5/5 (33)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.De EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Calificación: 4.5 de 5 estrellas4.5/5 (110)
- Summary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisDe EverandSummary: It Didn't Start with You: How Inherited Family Trauma Shapes Who We Are and How to End the Cycle By Mark Wolynn: Key Takeaways, Summary & AnalysisCalificación: 5 de 5 estrellas5/5 (3)
- The Happiness Trap: How to Stop Struggling and Start LivingDe EverandThe Happiness Trap: How to Stop Struggling and Start LivingCalificación: 4 de 5 estrellas4/5 (1)
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeDe EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeCalificación: 4.5 de 5 estrellas4.5/5 (253)
- The Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsDe EverandThe Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsAún no hay calificaciones
- The Tennis Partner: A Doctor's Story of Friendship and LossDe EverandThe Tennis Partner: A Doctor's Story of Friendship and LossCalificación: 4.5 de 5 estrellas4.5/5 (4)