Documentos de Académico
Documentos de Profesional
Documentos de Cultura
Oxygen Cycle: Plants
Cargado por
Apam BenjaminTítulo original
Derechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Oxygen Cycle: Plants
Cargado por
Apam BenjaminCopyright:
Formatos disponibles
Oxygen cycle
The Oxygen cycle is the biogeochemical cycle that describes the movement of oxygen within its three main reservoirs: the atmosphere (air), the total content of biological matter within the biosphere (the global sum of all ecosystems), and the lithosphere (Earth's crust). The continual interchange of oxygen between the atmosphere and the water, the plants and animals and mineral matter is called the oxygen cycle. Failures in the oxygen cycle within the hydrosphere (the combined mass of water found on, under, and over the surface of a planet) can result in the development of hypoxic zones. The main driving factor of the oxygen cycle is photosynthesis, which is responsible for the modern Earth's atmosphere and life. Importance of oxygen cycle Oxygen cycle is critical to both our health and the health of our environment. We need oxygen for respiration. The oxygen that we breathe oxidises the sugars in the food to generate energy. During this process carbon dioxide is released in the atmosphere.
Humans need oxygen to breathe Oxygen is needed for decomposition of organic waste. Water can dissolve oxygen and it is this dissolved oxygen that supports aquatic life.
The processes of Oxygen cycle Plants Plants mark the beginning of the oxygen cycle. Plants are able to use the energy of sunlight to convert carbon dioxide and water into carbohydrates and oxygen in a process called photosynthesis. During the day, plants hold onto a bit of the oxygen which they produced in photosynthesis and use that oxygen to break down carbohydrates. But in order to maintain their metabolism and continue respiration at night, the plants must absorb oxygen from the air and give off carbon dioxide just as animals do. Even though plants produce approximately ten times as much oxygen during the day as they consume at night, the night-time consumption of oxygen by plants can create low oxygen conditions in some water habitats. Water Oxygen in water is known as dissolved oxygen or DO. In nature, oxygen enters water when water runs over rocks and creates tremendous amounts of surface area. The high surface area allows oxygen to transfer from the air into the water very quickly.
1
When the water in a stream enters a pond, microorganisms in the pond begin to metabolize (break down) organic matter, consuming oxygen in the process. This is another form of oxygen cycle - oxygen enters water in rapids and leaves water in pools. Oxygen uptake rate (O.U.R.) is the rate at which oxygen is consumed by living organisms in the water. Since organisms are constantly using up oxygen in the water and oxygen is constantly reentering the water from the air, the amount of oxygen in water remains relatively constant. In a healthy ecosystem, the rates of oxygen transfer (being used up) and oxygen uptake are balanced in the water. Organisms Last are the organisms of the world. They use oxygen in many forms. Their role in the cycle begins with carbon dioxide in the atmosphere. Plants take in that carbon dioxide and combine it with water to create sugars and oxygen molecules. Animals breathe that oxygen and both plants and animals use the sugars for energy. Through the process of metabolism, the sugars are broken down into water and carbon dioxide. Then the cycle begins again. Reservoirs of oxygen By far the largest reservoir of Earth's oxygen is within the silicate and oxide minerals of the crust and mantle (99.5%). Only a small portion has been released as free oxygen to the biosphere (0.01%) and atmosphere (0.36%). The main source of atmospheric oxygen is photosynthesis, which produces sugars and oxygen from carbon dioxide and water:6CO2 + 6H2O + energy C6H12O6 + 6O2 Photosynthesizing organisms include the plant life of the land areas as well as the phytoplankton of the oceans. The tiny marine cyanobacterium Prochlorococcus was discovered in 1986 and accounts for more than half of the photosynthesis of the open ocean. [1] An additional source of atmospheric oxygen comes from photolysis, whereby high energy ultraviolet radiation breaks down atmospheric water and nitrous oxide into component atoms. The free H and N atoms escape into space leaving O2 in the atmosphere: 2H2O + energy 4H + O2 2N2O + energy 4N + O2 The main way oxygen is lost from the atmosphere is via respiration and decay, mechanisms in which animal life and bacteria consume oxygen and release carbon dioxide. The lithosphere also consumes oxygen. An example of surface weathering chemistry is formation of iron-oxides (rust):
4FeO + O2 2Fe2O3
Ozone The presence of atmospheric oxygen has led to the formation of ozone (O3) and the ozone layer within the stratosphere. The ozone layer is extremely important to modern life as it absorbs harmful ultraviolet radiation: O2 + uv energy 2O O + O2 O3 Oxygen Cycle and environment
Oxygen cycle plays a vital role in decomposition of organic waste. When dead tissue (carbon compounds) decays by a combination of oxidation and microorganism decay, carbon dioxide is released back to the atmosphere. A slower cycle occurs whenever mineral matter is oxidised, such as in the formation of rocks. The content of biodegradable substances in waste waters is expressed by a special index called "biological oxygen demand" (BOD), representing the amount of oxygen needed by aerobic bacteria to decompose the waste. With not enough oxygen available for these bacteria, for example, to waste much in a body of water, they die and anaerobic bacteria that do not need oxygen take over.
These bacteria change waste material into H2S and other poisonous and foul-smelling substances. Maintaining the Oxygen Cycle With the increase in the level of atmospheric pollution and large scale deforestation, experts worry about disturbing the balance in the atmosphere -- there might come a time when the amount of carbon dioxide is very large and there are very few plants and trees to convert it into Oxygen. This crisis will not know international boundaries. It is imperative that we plant more trees and reduce vehicular and industrial pollution.
También podría gustarte
- Chapter 7 Ionic and Metallic BondingDocumento22 páginasChapter 7 Ionic and Metallic Bondingapi-256257174100% (1)
- Earth Science QuizDocumento2 páginasEarth Science QuizAdrian Jaja LeungAún no hay calificaciones
- Atmosphere PDFDocumento6 páginasAtmosphere PDFAnonymous 4Jwgnyk5lVAún no hay calificaciones
- Essentials of Geology, 10e (Lutgens/Tarbuck/Tasa) Chapter 4 Volcanoes and Other Igneous ActivityDocumento15 páginasEssentials of Geology, 10e (Lutgens/Tarbuck/Tasa) Chapter 4 Volcanoes and Other Igneous Activitythuaiyaalhinai100% (1)
- ACTIVITY-3 Principles of GeologyDocumento5 páginasACTIVITY-3 Principles of GeologyRogemar PetalloAún no hay calificaciones
- Quiz #2Documento2 páginasQuiz #2Raphonzel AlvaradoAún no hay calificaciones
- Science 9 ReviewerDocumento2 páginasScience 9 ReviewerSamuel Arthur G. DomingoAún no hay calificaciones
- Water Cycle QuizDocumento4 páginasWater Cycle Quizapi-289664944Aún no hay calificaciones
- Properties of Ionic and Covalent Compounds LabDocumento1 páginaProperties of Ionic and Covalent Compounds LabKevonSingh1Aún no hay calificaciones
- VOLCANO NotesDocumento17 páginasVOLCANO NotesAlexander LahipAún no hay calificaciones
- The Oxygen CycleDocumento25 páginasThe Oxygen CycleDeepakKumarJIAún no hay calificaciones
- 2nd Quarter Exam For Science 9Documento5 páginas2nd Quarter Exam For Science 9Jocelyn MarmolAún no hay calificaciones
- Environmental Science Unit 2 TestDocumento5 páginasEnvironmental Science Unit 2 TestChemistry ClassAún no hay calificaciones
- Chemistry Particulate Nature of MatterDocumento21 páginasChemistry Particulate Nature of MatterFandyAún no hay calificaciones
- Reaction Mechanism in Organic ReactionsDocumento26 páginasReaction Mechanism in Organic Reactionspunt3yAún no hay calificaciones
- Atomic StructureDocumento50 páginasAtomic StructureJanette Cosip Gabo100% (1)
- Geology QuestionsDocumento14 páginasGeology Questionsanon_2257765420% (1)
- Earth Science Final AssessmentDocumento3 páginasEarth Science Final Assessmentapi-385958212Aún no hay calificaciones
- DRRR PPT 5.2Documento22 páginasDRRR PPT 5.2Jeynica Mie Ciancele LerdonAún no hay calificaciones
- Science 8 Diagnostic Test - Docx NewDocumento5 páginasScience 8 Diagnostic Test - Docx NewAtilrep Sailep TocilAún no hay calificaciones
- Syllabus Earth and Life ScienceDocumento2 páginasSyllabus Earth and Life ScienceAligora JoAún no hay calificaciones
- Second Quarter G9 TestDocumento3 páginasSecond Quarter G9 TestbryanAún no hay calificaciones
- Volcanoes RevisionDocumento15 páginasVolcanoes Revision3alliumcourtAún no hay calificaciones
- CH8 6 Volume and Moles Avogadros Laws GOB Structures 5th EdDocumento17 páginasCH8 6 Volume and Moles Avogadros Laws GOB Structures 5th EdJayr Linsangan100% (1)
- 2nd Departmental TestDocumento5 páginas2nd Departmental TestMelody Delleva BarcialAún no hay calificaciones
- Science 10 Summative Test q1 Module 1Documento4 páginasScience 10 Summative Test q1 Module 1Anafemolyn NingascaAún no hay calificaciones
- Mineral Rock TestDocumento8 páginasMineral Rock Testapi-331293056Aún no hay calificaciones
- q1 Mod6 HeatAndTemperature v2Documento29 páginasq1 Mod6 HeatAndTemperature v2MiyawiAún no hay calificaciones
- Batangas State UniversityDocumento2 páginasBatangas State UniversityAndrew Van RyanAún no hay calificaciones
- Evelyn Canonce LAS WK 3Documento8 páginasEvelyn Canonce LAS WK 3malouAún no hay calificaciones
- MagmaDocumento2 páginasMagmaKaren DellosaAún no hay calificaciones
- Chapter 2 - The Particulate Nature of MatterDocumento3 páginasChapter 2 - The Particulate Nature of MatterMahad AsimAún no hay calificaciones
- Fluid Pressure Quiz 2019Documento2 páginasFluid Pressure Quiz 2019Hermy E. Feliciano0% (1)
- Boyles Law WorksheetDocumento2 páginasBoyles Law WorksheetAbe Estrada EnanoAún no hay calificaciones
- Grade 10 Diagnostic TestDocumento4 páginasGrade 10 Diagnostic TestJr Capanang100% (1)
- DLL Q1Week2Documento3 páginasDLL Q1Week2Wendz ArominAún no hay calificaciones
- EFFECTS OF VOLCANIC ERUPTION - PPSXDocumento19 páginasEFFECTS OF VOLCANIC ERUPTION - PPSXMyla Balingit AdiAún no hay calificaciones
- Chem2 QuizDocumento32 páginasChem2 QuizCheng TamayoAún no hay calificaciones
- 11111Documento4 páginas11111Jezha Mae VertudazoAún no hay calificaciones
- DIVERGENT BOUNDARY (Divergence of Plates) Divergent Boundaries Occur When Two PlatesDocumento5 páginasDIVERGENT BOUNDARY (Divergence of Plates) Divergent Boundaries Occur When Two Platesdaisy sorianoAún no hay calificaciones
- Earth SubsystemDocumento6 páginasEarth SubsystemXenia Mae FloresAún no hay calificaciones
- Balancing Equations PDFDocumento6 páginasBalancing Equations PDFFeli CiaAún no hay calificaciones
- Atomic Theory PowerpointDocumento25 páginasAtomic Theory PowerpointRolly EgasAún no hay calificaciones
- Report On Geology About Malekhu AreaDocumento65 páginasReport On Geology About Malekhu Areabimal0% (1)
- Science 9 ReviewerDocumento7 páginasScience 9 ReviewerArgie MabagAún no hay calificaciones
- Continental Drift and Plate Tectonics Part 1Documento5 páginasContinental Drift and Plate Tectonics Part 1Jakie UbinaAún no hay calificaciones
- Quiz On Electromagnetism PDFDocumento1 páginaQuiz On Electromagnetism PDFMegAún no hay calificaciones
- Factor Afffecting ClimateDocumento38 páginasFactor Afffecting ClimatePrincess Alyssa BarawidAún no hay calificaciones
- The Richness of The Philippines in Terms of Mineral Resources Is Being Attributed To Its Location in The So-CalledDocumento3 páginasThe Richness of The Philippines in Terms of Mineral Resources Is Being Attributed To Its Location in The So-CalledNancy AtentarAún no hay calificaciones
- 1 - The Origin of The UniverseDocumento19 páginas1 - The Origin of The UniverseCAMILLE LOPEZAún no hay calificaciones
- Inawayan National High School - Darong Extension Science 7 Semi - Finals I. DIRECTIONS: Choose The Letter of The Best Answer. Write Your Answers inDocumento3 páginasInawayan National High School - Darong Extension Science 7 Semi - Finals I. DIRECTIONS: Choose The Letter of The Best Answer. Write Your Answers inRodel Camposo100% (1)
- Metals and Metallurgical PrinciplesDocumento13 páginasMetals and Metallurgical PrinciplesManoj KhanalAún no hay calificaciones
- DLL On Effects of Electromagnetic Waves (MANELYN P. V.)Documento3 páginasDLL On Effects of Electromagnetic Waves (MANELYN P. V.)Silver Ritz100% (1)
- 3rd Monthly Exam Grade 10Documento4 páginas3rd Monthly Exam Grade 10MARY ROSEAún no hay calificaciones
- Chapter Test On Sound WavesDocumento1 páginaChapter Test On Sound WavesryanmanubagAún no hay calificaciones
- Grade 7 Module 2 Earth SciDocumento10 páginasGrade 7 Module 2 Earth SciMarianne MendozaAún no hay calificaciones
- 4th Summative Test Earth N SpaceDocumento5 páginas4th Summative Test Earth N SpaceAbigail Fernandez BatallerAún no hay calificaciones
- Oxygen CycleDocumento4 páginasOxygen CyclePradnya GoreAún no hay calificaciones
- The 5 Nutrient Cycles - Science Book 3rd Grade | Children's Science Education booksDe EverandThe 5 Nutrient Cycles - Science Book 3rd Grade | Children's Science Education booksAún no hay calificaciones
- Oxygen CycleDocumento5 páginasOxygen Cycleakms_Saif2521Aún no hay calificaciones
- Research ProposalDocumento6 páginasResearch ProposalApam BenjaminAún no hay calificaciones
- Lecture7 Module2 Anova 1Documento10 páginasLecture7 Module2 Anova 1Apam BenjaminAún no hay calificaciones
- Nitrogen Cycle: Ecological FunctionDocumento8 páginasNitrogen Cycle: Ecological FunctionApam BenjaminAún no hay calificaciones
- Cocoa (Theobroma Cacao L.) : Theobroma Cacao Also Cacao Tree and Cocoa Tree, Is A Small (4-8 M or 15-26 FT Tall) EvergreenDocumento12 páginasCocoa (Theobroma Cacao L.) : Theobroma Cacao Also Cacao Tree and Cocoa Tree, Is A Small (4-8 M or 15-26 FT Tall) EvergreenApam BenjaminAún no hay calificaciones
- Lecture8 Module2 Anova 1Documento13 páginasLecture8 Module2 Anova 1Apam BenjaminAún no hay calificaciones
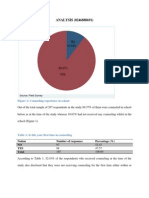
- ANALYSIS (0246888691) : Figure 1: Counseling Experience in SchoolDocumento8 páginasANALYSIS (0246888691) : Figure 1: Counseling Experience in SchoolApam BenjaminAún no hay calificaciones
- Multivariate Analysis of Variance (MANOVA) : (Copy This Section To Methodology)Documento8 páginasMultivariate Analysis of Variance (MANOVA) : (Copy This Section To Methodology)Apam BenjaminAún no hay calificaciones
- Lecture6 Module2 Anova 1Documento10 páginasLecture6 Module2 Anova 1Apam BenjaminAún no hay calificaciones
- PLM IiDocumento3 páginasPLM IiApam BenjaminAún no hay calificaciones
- PLMDocumento5 páginasPLMApam BenjaminAún no hay calificaciones
- Lecture4 Module2 Anova 1Documento9 páginasLecture4 Module2 Anova 1Apam BenjaminAún no hay calificaciones
- Lecture5 Module2 Anova 1Documento9 páginasLecture5 Module2 Anova 1Apam BenjaminAún no hay calificaciones
- Finish WorkDocumento116 páginasFinish WorkApam BenjaminAún no hay calificaciones
- Hosmer On Survival With StataDocumento21 páginasHosmer On Survival With StataApam BenjaminAún no hay calificaciones
- Trial Questions (PLM)Documento3 páginasTrial Questions (PLM)Apam BenjaminAún no hay calificaciones
- Ajsms 2 4 348 355Documento8 páginasAjsms 2 4 348 355Akolgo PaulinaAún no hay calificaciones
- A Study On School DropoutsDocumento6 páginasA Study On School DropoutsApam BenjaminAún no hay calificaciones
- How Does Information Technology Impact On Business Relationships? The Need For Personal MeetingsDocumento14 páginasHow Does Information Technology Impact On Business Relationships? The Need For Personal MeetingsApam BenjaminAún no hay calificaciones
- ARIMA (Autoregressive Integrated Moving Average) Approach To Predicting Inflation in GhanaDocumento9 páginasARIMA (Autoregressive Integrated Moving Average) Approach To Predicting Inflation in GhanaApam BenjaminAún no hay calificaciones
- Personal Information: Curriculum VitaeDocumento6 páginasPersonal Information: Curriculum VitaeApam BenjaminAún no hay calificaciones
- Creating STATA DatasetsDocumento4 páginasCreating STATA DatasetsApam BenjaminAún no hay calificaciones
- Regression Analysis of Road Traffic Accidents and Population Growth in GhanaDocumento7 páginasRegression Analysis of Road Traffic Accidents and Population Growth in GhanaApam BenjaminAún no hay calificaciones
- 6th Central Pay Commission Salary CalculatorDocumento15 páginas6th Central Pay Commission Salary Calculatorrakhonde100% (436)
- How To Write A Paper For A Scientific JournalDocumento10 páginasHow To Write A Paper For A Scientific JournalApam BenjaminAún no hay calificaciones
- MSC Project Study Guide Jessica Chen-Burger: What You Will WriteDocumento1 páginaMSC Project Study Guide Jessica Chen-Burger: What You Will WriteApam BenjaminAún no hay calificaciones
- Banque AtlanticbanDocumento1 páginaBanque AtlanticbanApam BenjaminAún no hay calificaciones
- Asri Stata 2013-FlyerDocumento1 páginaAsri Stata 2013-FlyerAkolgo PaulinaAún no hay calificaciones
- l.4. Research QuestionsDocumento1 páginal.4. Research QuestionsApam BenjaminAún no hay calificaciones
- Project ReportsDocumento31 páginasProject ReportsBilal AliAún no hay calificaciones
- Caltech RefDocumento308 páginasCaltech RefSukrit ChatterjeeAún no hay calificaciones
- ResultofCFAPMSAExaminations Winter2022Documento62 páginasResultofCFAPMSAExaminations Winter2022Nauman NafeesAún no hay calificaciones
- GST 101 Exam Past QuestionsDocumento6 páginasGST 101 Exam Past QuestionsBenjamin Favour100% (2)
- Su Jok, Twist Therapy AND Smile MeditationDocumento7 páginasSu Jok, Twist Therapy AND Smile MeditationprateekAún no hay calificaciones
- Argumentative Essay Project DescriptionDocumento5 páginasArgumentative Essay Project DescriptionKaren Jh MoncayoAún no hay calificaciones
- (Paper-2) 20th Century Indian Writing: Saadat Hasan Manto: Toba Tek SinghDocumento18 páginas(Paper-2) 20th Century Indian Writing: Saadat Hasan Manto: Toba Tek SinghApexa Kerai67% (3)
- PlayDocumento121 páginasPlayellennelleAún no hay calificaciones
- Character Formation 1: Nationalism and PatriotismDocumento11 páginasCharacter Formation 1: Nationalism and Patriotismban diaz100% (1)
- QuotesDocumento12 páginasQuotesflowerkmAún no hay calificaciones
- Man Is Made by His BeliefDocumento2 páginasMan Is Made by His BeliefLisa KireechevaAún no hay calificaciones
- Music in The United KingdomDocumento33 páginasMusic in The United KingdomIonut PetreAún no hay calificaciones
- The ReformationDocumento20 páginasThe ReformationIlyes FerenczAún no hay calificaciones
- Task Basis JurisprudenceDocumento10 páginasTask Basis JurisprudenceKerwin LeonidaAún no hay calificaciones
- Strategi Pencegahan Kecelakaan Di PT VALE Indonesia Presentation To FPP Workshop - APKPI - 12102019Documento35 páginasStrategi Pencegahan Kecelakaan Di PT VALE Indonesia Presentation To FPP Workshop - APKPI - 12102019Eko Maulia MahardikaAún no hay calificaciones
- PED100 Mod2Documento3 páginasPED100 Mod2Risa BarritaAún no hay calificaciones
- Roman Villas at Tor Marancia and CentocelleDocumento10 páginasRoman Villas at Tor Marancia and CentocelleIgor ĆirkovićAún no hay calificaciones
- FreeMarkets: Procurement & Outsourcing StrategiesDocumento44 páginasFreeMarkets: Procurement & Outsourcing StrategiesFarhaad MohsinAún no hay calificaciones
- The Bible Does Not Condemn Premarital SexDocumento16 páginasThe Bible Does Not Condemn Premarital SexKeith502100% (3)
- Spyderco Product Guide - 2016Documento154 páginasSpyderco Product Guide - 2016marceudemeloAún no hay calificaciones
- Ccounting Basics and Interview Questions AnswersDocumento18 páginasCcounting Basics and Interview Questions AnswersAamir100% (1)
- Bird Beak ActivityDocumento4 páginasBird Beak Activityapi-314222661Aún no hay calificaciones
- Midterm Decision Analysis ExercisesDocumento5 páginasMidterm Decision Analysis ExercisesAYLEN INJAYAAún no hay calificaciones
- Rediscovering The True Self Through TheDocumento20 páginasRediscovering The True Self Through TheManuel Ortiz100% (1)
- Practice Makes Perfect Basic Spanish Premium Third Edition Dorothy Richmond All ChapterDocumento67 páginasPractice Makes Perfect Basic Spanish Premium Third Edition Dorothy Richmond All Chaptereric.temple792100% (3)
- Professional Education Pre-Licensure Examination For TeachersDocumento12 páginasProfessional Education Pre-Licensure Examination For TeachersJudy Mae ManaloAún no hay calificaciones
- Software Project Management PDFDocumento125 páginasSoftware Project Management PDFUmirAún no hay calificaciones
- Olinger v. The Church of Jesus Christ of Latter Day Saints Et Al - Document No. 1Documento4 páginasOlinger v. The Church of Jesus Christ of Latter Day Saints Et Al - Document No. 1Justia.comAún no hay calificaciones
- The Role of Personalization, Engagement and Trust in Online CommunitiesDocumento17 páginasThe Role of Personalization, Engagement and Trust in Online CommunitiesAbiAún no hay calificaciones
- SAP CRM Tax ConfigurationDocumento18 páginasSAP CRM Tax Configurationtushar_kansaraAún no hay calificaciones
- Planet Maths 5th - Sample PagesDocumento30 páginasPlanet Maths 5th - Sample PagesEdTech Folens48% (29)