Documentos de Académico
Documentos de Profesional
Documentos de Cultura
Indicators List
Cargado por
agadaltzaDerechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Indicators List
Cargado por
agadaltzaCopyright:
Formatos disponibles
A Amido black 10 B (redox) Dissolve 0.2 g dye in 100 ml of water color change = yellowish-brown to blue Alizarin Dissolve 0.1g.
in 100 ml. of 95% ethanol. pH =10.1 - 12.1; color change = red to purple Alizarin yellow Dissolve 0.1g. in 100 ml. of water. pH =10.0 - 12.1; color change = light yellow to brownish yellow Alizarin sulphonic acid Na salt Dissolve 0.1g. in 100 ml. water or in 100 ml 1:1 ethanol and water. pH =4.3 - 6.3; color change = yellow to violet Alkali blue Dissolve 0.1g. in 100 ml. of 95% ethanol. pH =9.4 - 14.0; color change = violet to pink B 2.2'Bipyridin (iron(II) complex (redox) dissolve 0.695 g of FeSO4.7H2O and 1.171 g of 2.2'bipyridin in 100 ml of water color change = pale blue to red Brillant cresyl blue (redox) dissolve 0.5 g in 100 ml of water or ethanol (96 %) color change = blue to colorless Brilliant green Dissolve 0.1g. in 100 ml. of water. pH =0.0 - 2.6; color change = yellow to green Bromochlorophenol blue Dissolve 0.1g. in 100 ml. of 20% ethanol, or dissolve 0.04g in 0.69 ml of 0.1M sodium hydroxide and make up to 100 ml with water. pH =3.0 - 4.6; color change = yellow to blue-violet Bromocresol green Dissolve 0.1g. in 100 ml. of 20% ethanol, or dissolve 0.04g in 0.58 ml of 0.1Msodium hydroxide and make up to 100 ml with water. pH =3.8 - 5.4; color change = yellow to blue Bromocresol purple Dissolve 0.1g. in 100 ml. of 20% ethanol, or dissolve 0.4g in 0.74 ml of 0.1M sodium hydroxide and make up to 100 ml. pH =5.2 - 6.8; color change = yellow to purple Bromophenol blue Dissolve 0.1g. in 100 ml. of 20% ethanol, or dissolve 0.04g in 0.6 ml of 0.1M sodium hydroxide and make up to 100 ml. pH =3.0 - 4.6; color change = yellow to blue-violet
Bromophenol red Dissolve 0.1g. in 100 ml. of 20% ethanol, or dissolve 0.04g in 0.94 ml of 0.1M sodium hydroxide and make up to 100 ml. pH =5.2 - 6.8; color change = orange yellow to purple Bromothymol blue Dissolve 0.1g. in 100 ml. of 20% ethanol. Bromo-oxylenol blue Dissolve 0.1g. in 100 ml. of 95% ethanol. pH =5.7 - 7.5; color change = orange yellow to blue C Cacotheline saturated color change = yellow to red-violet Calcon also known as Solochrome Dark Blue or Eriochrome blue black R (metal-ion EDTA) sodium 1-(2- hydroxy-1-naphthylazo)-2-naphthol-4sulphonate, Colour I ndex No.202; Dissolve 0.2g of the dyestuff in 50ml. of methanol. The colour change is from pink to pure blue. Calmagite 1-(1-hydroxy-4-methyl-2-phenylazo)-2-naphthol-4sulphonic acid (IX) (metal-ion EDTA) Can be substituted for Eriochrome Black T without change in the experimental procedures for calcium and magnesium.It has same colour change, which is clearer and sharper, and aqueous solutions of the indicator are almost stable indefinitely. Dissolve 0.05g of calmagiite in 100ml. of water. The indicator is stable for at least 12 months when stored in a polythene bottle and in the dark. Chlorophenol red Dissolve 0.1g. in 100 ml. of 20% ethanol, or dissolve 0.04g in 0.94 ml of 0.1M sodium hydroxide and make up to 100 ml. pH =4.8 - 6.4; color change = yellow to purple Congo red Dissolve 0.2g. in 100 ml. of water. pH =3.0 - 5.2; color change = blue to orange-yellow Cresol purple Dissolve 0.04g. in 100 ml. of 20% ethanol, or dissolve 0.04g in 0.86 ml of 0.1M sodium hydroxide and make up to 100 ml. pH =7.4 - 9.0; color change = yellow to purple Cresol red Dissolve 0.1g. in 100 ml. of 50% ethanol, or dissolve 0.04g in 1.05 ml of 0.1M sodium hydroxide and make up to 100 ml. pH =7.0 - 8.8; color change = yellow to purple
Cresol red Dissolve 0.1g. in 100 ml. of 20% ethanol, or dissolve 0.04g in 1.05 ml of 0.1M sodium hydroxide and make up to 100 ml. pH =0.2 - 1.8; color change = red to yellow m-Cresol purple Dissolve 0.04g. in 100 ml. of 20% ethanol, or dissolve 0.04g in 1.05 ml of 0.1M sodium hydroxide and make up to 100 ml. pH =1.2 - 2.8; color change = red to yellow Crystal violet Dissolve 0.1g. in 100 ml. of 70% ethanol. pH =0.8 - 2.6; color change = yellow to blue-violet D Dibromopyrogallol sulphone phthalein (VIII) (metal-ion EDTA) or Bromopyrogallol Red Dissolve 0.05g of the reagent in 100ml. of 50% ethanol. The indicator is coloured orange-yellow in strongly acid solutions,; claret red in nearly neutral solutions; and violet to blue in basic solutions. Dichlorofluorescein: (adsorption) Dissolve 0.1g in 60 - 70 % ethanol, or Dissolve 0.1g of dichlorofluoroceinate in 100ml water. 2.6-Dichlorophenolin-dophenol sodiumsalt (dihydrate) (redox) dissolve 0.02 g in 100 ml of water color change = blue to colorless Di-iododimethylfluorescein: (adsorption) Dissolve 1g in 70 5 ethanol. 4-(Dimethylamino) azobenzene Dissolve 0.1 to 1.5 g. in 100 ml. of 90% ethanol. pH =2.9 - 4.0; color change = red to yellow-orange 3.3'-Dimethylnahthidine(4.4'-Diamino-3.3-dimethyl1.1'-binaphthaline) (redox) dissolve 1.0 g in 100 ml of glacial acetic acid color change = purple-red to colorless N,N-Dimethyl-1.4-phenylenediammonium dichloride (redox) dissolve 0.02 g in 100 ml of water color change = dark-blue to colorless 2,5-Dinitrophenol Dissolve 0.05 to 0.1g. in 100 ml. of 70% ethanol. pH =4.0 - 5.8; color change = colorless to yellow 2,4-Dinitrophenol Dissolve 0.1g. in 100 ml. of 70% ethanol. pH =2.8 - 4.7; color change = colorless to yellow Diphenylamine: Dissolve 1g in 100 ml conc. H2SO4 color change = blue-violet to colorless
Diphenylamine-4-sulfonic acid barium salt (redox) Dissolve 0.2g in 100 ml water color change = red-violet to colorless Diphenylamine-p-sulphonic acid (Na salt): (redox) Dissolve 0.2g in 100 ml water. Diphenylbenzidine: (redox) Dissolve 1g in 100 ml conc.H2SO4 color change = violet to colorless Diphenylcarbazide: (adsorption) Dissolve 0.1g in 100 ml ethanol. Diphenylcarbazone: (adsorption) Dissolve 0.1g in 100 ml ethanol or iso-propyl alcohol. E Eosin bluish Dissolve 0.1g. in 100 ml. of water. pH =1.4 - 2.4; color change = colorless to pink fluorescence Eosin: (adsorption) Dssolve 0.1g in 100ml 70 % ethanol, or 0.1g of the sodium salt in 100 ml water. Eosin yellowish Dissolve 0.1g. in 100 ml. of water. pH =0.0 - 3.0; color change = yellow to green fluorescence Epsilon blue Dissolve 0.1g. in 100 ml. of water. pH =11.6 - 13.0; color change = orange to violet Eriochrome Black T (metal-ion EDTA, also known as Solochrome black T or Mordant black) Sodium 1-(1-hydroxy-2-naphthalyzo)-6-nitro-2naphthol-4-sulphonate (1); also known as Solochrome Black T or WDFA or No. 2 in the Colour Index. Not recommended for titration of solutions more acidic than Ph 6.5. Dissolve 0.2 g of the dyestuff in 15ml. of ethanolamine and add 5ml of absolute ethanol to reduce the viscosity; the reagent is stable for several months. A 0.4% solution of the pure dye in methanol may last for about a month. Colour change is from blue to red. Eriochrome blue black (See Calcon) Eriochrome Red B sodium salt of 4-(2-hydroxy-4-sulpho-1-naphthylazo)-3methyl-1-phenyl-2-pyrazolin-5-one (IX)---a pyrazolone azo-B-naphthol dyestuff. (metal-ion EDTA) Dissolve 0.1g of the dyestuff in 50ml, ethanol. It is stable indefinitely. The colour change from pink to pale yellow is almost instantaneous at about 80oC.
Erythrosin B Dissolve 0.1g. in 100 ml. of water. pH =0.0 - 3.6; color change = orange to red FGHIJKL Fast Sulphon Black F (metal ion EDTA) Sodium salt of 1-hydrovy-8-(2-hydroxynaphthylazo)-2(solphonaphtylazo)-3,6-disulphonic acid (V) The indicator solution is 0.5% solution in water. Specific colour change for copper is from magenta to pale blue to bright green. Ferric indicator: (adsorption) Use a saturated Ammonium Ferric sulphate soln. (~ 40%), add a few drops of 6M nitric acid, and use 1 ml for each titration. Ferroin: (redox) Make a 0.025M in water. or Dissolve 0.7g FeSO4.7H2O and 1.5g 1,10 o-phenanthroline in 100 ml water. color change = blue to orange-red Fluorescein: (adsorption) Dissolve 0.2g in 100 ml 70 % alcohol, or Dissolve 0.2g of sodium fluorocienate in 100 ml water. HHSNNA (metal-ion EDTA) See Patton and Reeder's Indigo carmine Dissolve 0.25g. in 100 ml. of 50% ethanol, or dissolve 1g in 100ml of water. pH =11.5 - 13.0; color change = blue to yellow Indigo carmine (Indigo disulfonate disodium salt) (redox) Dissolve 0.5g in 100 ml water color change = blue to yellowish Litmus Dissolve 4g. in 100 ml. of water. pH =5.0 - 8.0; color change = red to blue Note on Litmus:You can purify the commercial litmus as follows: Digest 10g. of the litmus with 35ml.of rectified spirit on a water bath for about 1 hr and decant the alcohol.; repeat this process twice. Extract the residue several times with water and allow to stand for several days. Decant or siphon off the clear extract. This is of suitable concentration for most purposes. Bromo-cresol purple or Bromo-thymol blue are excellent substitutes for litmus. Azolitmin is the pure litmus colouring matter. Dissolve 0.1g. in 100ml of water. M Malachite green oxalate Dissolve 0.1g. in 100 ml. of water. pH =0.0 - 2.0; color change = yellow to green-blue
N-Methlydiphenylamine-p-solphonic acid, (Na salt): (redox) Dissolve 0.1g in 100 ml water. Methylene blue (redox) Dissolve 0.1 to 0.5g in 100 ml water Methyl green Dissolve 0.1g. in 100 ml. of water. pH =0.1 - 2.3; color change = yellow to blue Methyl orange Dissolve 0.05g. of the sodium salt in 100 mls. of water, add 8 mls..of 0.1M hydrochloric acid, and filter if necessary, or Dissolve 0.05g. of the free acid in 100 ml. water, and filter the solution if a precipitate forms. pH =3.1 - 4.4; color change is pink/red towards yelloworange Screened methyl orange Dissolve 1g. of methyl orange and 1.4g. of xylene cyanol FF in 200ml. water and make up to 500 ml. vol. with ethanol. The purpose of a screened or mixed indicator is to produce a more pronounced colour change at the end point. These types of indicators consist of either a mixture of two indicators or a mixture of an indicator and an inert dye. It changes colour at pH 3.8 -- 4.1 from violet to green. Methyl red Dissolve 0.1g. of the Na salt in 100ml. of water, or dissolve in 30ml. of alcohol and dilute to 100ml. vol. with water. pH =4.4 - 6.2; color change = red to yellow-orange Methyl violet (Methyl Violet 10B or Gentian Violet) Dissolve 0.1g. in 100 ml. of 20% ethanol. pH =0.1 - 2.7; color change = yellow to violet Methyl yellow Dissolve 0.1g. of the indicator in 100 ml of alcohol. Mixed indicator Dissolve 0.2g of methyl red and 0.1g of methyl blue or bromocresol green in 100 ml 95% ethanol pH =4.3 - 5.2; color change = green to pink(ph 4.5) Mordant black (See Eriochrome black T) Murexide (metal-ion EDTA) This is the Ammonium salt of purpuric acid. Suspend 0.5g of the powdered dyestuff in water, shake thoroughly and allow to settle. The saturated supernatant is used as the indicator. The colour change is towards a blue endpoint. N 1-Napltolphthalein Dissolve 0.1g. in 100 ml. of 95% ethanol. pH =7.1 - 8.3; color change = brownish to blue-green
Neutral red Dissolve 0.3g. in 100 ml. of 70% ethanol. pH =6.8 - 8.0; color change = blue-red to orange-yellow Neutral red (redox) Dissolve 0.5g in 100 ml of 95% ethanol color change = violet-red to colorless Nile blue (sulphate) (redox) Dissolve 0.1g in 100 ml water color change = blue-red to colorless 3-Nitrophenol Dissolve 0.3g. in 100 ml. of 95% ethanol, or 0.08 g in 100 ml water. pH =6.6 - 8.6; color change = colorless to yellow-orange 4-Nitrophenol Dissolve 0.2g. in 100 ml. of 95% ethanol, or 0.08g in 100 ml water. pH =5.4 - 6.8; color change = yellow to violet OP Patton and Reeders indicator (metal-ion EDTA) 2-hydroxy-1-(2-hydroxy-4-sulpho-1-naphthylazo)-3naphthoic acid (111) Also known by the abbreviated name HHSNNA. The dyestuff is thoroughly mixed with 100 times its weight of sodium sulphate, and 1g of this mix is used for each titration. Used in the direct titration of calcium, particularly in the presence of magnesium, pH range 12-14. A sharp colour change is obtained from wine red to pure blue. Phenolphthalein Dissolve 0.5 g. of the reagent in 50ml.of alcohol and add 50ml of water with stirring. Filter if a precipitate forms or Dissolve 1g. of the dry indicator in 80 mls.ethylene glycol monoethyl ether (cellosolve) b.p.135oC, and dilute to 100ml. with distilled water.: the loss by evaporation is less by this preparation. Phenol red Dissolve 0.1g. in 100 ml. of 20% ethanol, or dissolve 0.04g in 1.13 ml of 0.1M sodium hydroxide and make up to 100 ml. pH =6.4 - 8.2; color change = yellow to red-violet Pentamethoxytriphenyl carbinol Dissolve 0.1 g in 100 ml of 95% ethanol pH =1.2 - 3.2; color change = red to colorless 1.10-Phenanthroline (monohydrate) (redox) Dissolve 0.695 g of FeSO4 x 7H2O and 1.487 g of 1.10Phenanthroline in 100 ml of water color change = pale-blue to red
N-Phenylanthranilic acid: (redox) Dissolve 0.1 g in 5 ml of 0.1M sodium hydroxide and make up to 100 ml with water. color change = purple-red to colorless Picric acid Dissolve 0.1 g in 100 ml of 20% ethanol pH =0.2 - 1.0; color change = colorlass to yellow Pyrocatechol Violet Pyrocatechol sulphone phthalein (VII); Catechol violet. (metal-ion EDTA) Dissolve 0.1g of the dyestuyy in 100ml.of water. This solution is stable for several weeks. Colour change is progressive, from yellow yo blue to green. QRS Quinaldine red Dissolve 0.1 g in 100 ml of 60% ethanol pH =1.4 - 3.2; color change = colorless to pink Rhodamine: (adsorption) Dissolve 0.05 g in 100 ml water. Safranin (redox) Dissolve 0.5g in 100 ml water color change = blue-violet(acidic),brown (alkaline) to colorless T 4,5,6,7-Tetrabromo-phenolphthalein Dissolve 0.1 g in 100 ml of 95% ethanol pH =7.0 - 8.0; color change = colorless to purple 2.2':6.2"-Terpyridine (iron(II) complex) (redox) Dissolve 0.232 g FeSO4 x 7H2O and 0.389 g of 2.2':6.2terpyridine in 100 ml of water color change = pale-blue to red Tertrazine: (redox) Dissolve 0.5g in water and use 4 drops per titration. Thionine (redox) Dissolve 0.5g in 100 ml of 95% ethanol color change = violet to colorless Titan yellow Dissolve 0.1 g in 100 ml of 20% ethanol pH =12.0 - 13.0; color change = yellow to red Thymol blue Dissolve 0.04g. in 100 ml. of 20% ethanol, or dissolve 0.04g in 0.86 ml of 0.1M sodium hydroxide and make up to 100 ml with water. pH =8.0 - 9.6; color change = yellow to blue
Thymol blue Dissolve 0.04g. in 100 ml. of 20% ethanol, or dissolve 0.04g in 0.86 ml of 0.1M sodium hydroxide and make up to 100 ml with water. pH =1.2 - 2.8; color change = red to yellow Thymolphthalein Dissolve 0.1 g in 100 ml of 50% ethanol pH =9.3 - 10.5; color change = yellow to red Thymolphthalexone Thymolphthalein complexone (metal-ion EDTA) 3,3-bis-[n,n-(carboxymethyl)aminomethyl] thymolphthalein (X) Prepare a 0.5% solution in ethanol.alternatively, a finely ground mixture 1:100 with AR potassium nitrate may be used. Blue to colourless or slight pink in alkaline medium. Tropaeolin O Dissolve 0.1g. of the solid in 100 ml of water. Tropaeolin OO Dissolve 0.1g. of the solid in 100 ml of water. UVWXYZ Universal indicator (Full-range) Dissolve 0.026g thymol blue, 0.060g mrthyl red, 0.300g bromothymol blue, and 0.500g phenolphthalein in about 500ml ethanol. Add dilute NaOH until solution turns green (neutral), Color changes from strong acid to
alkaline: red to yellow to green (neutral) to blue to purple. Variamine Blue B (metal-ion EDTA) 4-methoxy-4-amino-diphenylamine The indicator solution is a 1% solution of the base in water. Ferric complex with EDTA - sharp change in redox potential - colourless to violet blue complex. Variamine blue salt B (redox) Dissolve 1.0 g in 100 ml of water or grind with sodium chloride or sodium sulfate anhydrous trituration color change = blue-violet(acidic),yellow (alkaline) to colorless p-Xylenol blue Dissolve 0.1g. in 100 ml. of 50% ethanol, or dissolve 0.04g in 0.98 ml of 0.1M sodium hydroxide and make up to 100 ml with water. pH =1.2 - 2.8; color change = red to yellow p-Xylenol blue Dissolve 0.1g. in 100 ml. of 50% ethanol, or dissolve 0.04g in 0.98 ml of 0.1M sodium hydroxide and make up to 100 ml with water. pH =8.0 - 9.6; color change = yellow to blue Xylenol Orange 3,3-bis[NN-di-(carboxymethyl)-aminomethyl]-ocresolsulphonephthalein (VI) Dissolve 0.5g of xylenol orange indicator in 100ml. of water. The solution is stable indeffinitely. Acid solutions are coloured lemon yellow and those of the metal complexes intensely red.
Indicator
Low pH color
Transition pH range
High pH color
Gentian violet (Methyl violet 10B)
yellow
0.02.0
blue-violet
Malachite green (first transition)
yellow
0.02.0
green
Malachite green (second transition)
green
11.614
colorless
Thymol blue (first transition)
red
1.22.8
yellow
Thymol blue (second transition)
yellow
8.09.6
blue
Methyl yellow
red
2.94.0
yellow
Bromophenol blue
yellow
3.04.6
purple
Congo red
blue-violet
3.05.0
red
Methyl orange
red
3.14.4
yellow
Screened methyl orange (first transition)
red
0.03.2
grey
Screened methyl orange (second transition)
grey
3.24.2
green
Bromocresol green
yellow
3.85.4
blue
Methyl red
red
4.46.2
yellow
Indicator
Low pH color
Transition pH range
High pH color
Azolitmin
red
4.58.3
blue
Bromocresol purple
yellow
5.26.8
purple
Bromothymol blue
yellow
6.07.6
blue
Phenol red
yellow
6.48.0
red
Neutral red
red
6.88.0
yellow
Naphtholphthalein
colorless to reddish
7.38.7
greenish to blue
Cresol Red
yellow
7.28.8
reddish-purple
Phenolphthalein
colorless
8.310.0
fuchsia
Thymolphthalein
colorless
9.310.5
blue
Alizarine Yellow R
yellow
10.212.0
red
También podría gustarte
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryCalificación: 3.5 de 5 estrellas3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Calificación: 4.5 de 5 estrellas4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe EverandNever Split the Difference: Negotiating As If Your Life Depended On ItCalificación: 4.5 de 5 estrellas4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaCalificación: 4.5 de 5 estrellas4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe EverandThe Little Book of Hygge: Danish Secrets to Happy LivingCalificación: 3.5 de 5 estrellas3.5/5 (399)
- Grit: The Power of Passion and PerseveranceDe EverandGrit: The Power of Passion and PerseveranceCalificación: 4 de 5 estrellas4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyCalificación: 3.5 de 5 estrellas3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeCalificación: 4 de 5 estrellas4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnDe EverandTeam of Rivals: The Political Genius of Abraham LincolnCalificación: 4.5 de 5 estrellas4.5/5 (234)
- Rise of ISIS: A Threat We Can't IgnoreDe EverandRise of ISIS: A Threat We Can't IgnoreCalificación: 3.5 de 5 estrellas3.5/5 (137)
- Shoe Dog: A Memoir by the Creator of NikeDe EverandShoe Dog: A Memoir by the Creator of NikeCalificación: 4.5 de 5 estrellas4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDe EverandThe Emperor of All Maladies: A Biography of CancerCalificación: 4.5 de 5 estrellas4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreCalificación: 4 de 5 estrellas4/5 (1090)
- Her Body and Other Parties: StoriesDe EverandHer Body and Other Parties: StoriesCalificación: 4 de 5 estrellas4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersCalificación: 4.5 de 5 estrellas4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceCalificación: 4 de 5 estrellas4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureCalificación: 4.5 de 5 estrellas4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaDe EverandThe Unwinding: An Inner History of the New AmericaCalificación: 4 de 5 estrellas4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)De EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Calificación: 4 de 5 estrellas4/5 (98)
- The Perks of Being a WallflowerDe EverandThe Perks of Being a WallflowerCalificación: 4.5 de 5 estrellas4.5/5 (2099)
- Choose The Correct. (Marks: 50)Documento37 páginasChoose The Correct. (Marks: 50)AmaanAún no hay calificaciones
- On Fire: The (Burning) Case for a Green New DealDe EverandOn Fire: The (Burning) Case for a Green New DealCalificación: 4 de 5 estrellas4/5 (73)
- Hobart Filler Metals CatalogDocumento244 páginasHobart Filler Metals CatalogBhrugu DhokaiAún no hay calificaciones
- Training The ABC's of Perfumery: Stephen V. Dowthwaite PerfumersWorldDocumento27 páginasTraining The ABC's of Perfumery: Stephen V. Dowthwaite PerfumersWorldSteve100% (16)
- Introduction About Steel FiberDocumento25 páginasIntroduction About Steel FiberVirupakshappa C Koti100% (4)
- EXAMPLE SIZING OWS Calculation Per API 421 PDFDocumento1 páginaEXAMPLE SIZING OWS Calculation Per API 421 PDFarnel_ado4412Aún no hay calificaciones
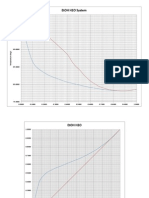
- Etoh H2o Txy Equil CurveDocumento3 páginasEtoh H2o Txy Equil CurveagadaltzaAún no hay calificaciones
- Temperature and Composition Data for EtOH H2O SystemDocumento8 páginasTemperature and Composition Data for EtOH H2O SystemagadaltzaAún no hay calificaciones
- Letter Overnight LabDocumento2 páginasLetter Overnight LabagadaltzaAún no hay calificaciones
- VolumeDocumento1 páginaVolumeagadaltzaAún no hay calificaciones
- Conversion UnitsDocumento2 páginasConversion UnitsagadaltzaAún no hay calificaciones
- CPI Terminologies 2013Documento13 páginasCPI Terminologies 2013agadaltzaAún no hay calificaciones
- Google Books Source For PLDocumento1 páginaGoogle Books Source For PLagadaltzaAún no hay calificaciones
- CHM131 MAC 2019 exam: Density, isotopes, balancing equationsDocumento4 páginasCHM131 MAC 2019 exam: Density, isotopes, balancing equationsijah rosmiAún no hay calificaciones
- Water Flushing Witnessing and TestDocumento3 páginasWater Flushing Witnessing and TestNeguib FarahAún no hay calificaciones
- An800 6Documento3 páginasAn800 6jcAún no hay calificaciones
- Introduction To Cell BiologyDocumento43 páginasIntroduction To Cell BiologyEllemrac GageloniaAún no hay calificaciones
- Msds Super Gloss Oil PaintDocumento3 páginasMsds Super Gloss Oil PaintMD AbdullahAún no hay calificaciones
- Material Data Sheet: Urban@plastum - CZ WWW - Plastum.czDocumento1 páginaMaterial Data Sheet: Urban@plastum - CZ WWW - Plastum.czDavis GAún no hay calificaciones
- Samanea Saman 8Documento7 páginasSamanea Saman 8Jesus Llorente mendozaAún no hay calificaciones
- The Effect of Different Fertilizers On Plant GrowthDocumento2 páginasThe Effect of Different Fertilizers On Plant GrowthVeerath தமிழன்0% (2)
- Stainless Steel Cable Tie GuideDocumento8 páginasStainless Steel Cable Tie GuideSathorn TumAún no hay calificaciones
- PCN ISI/ Appendix Z1 Issue 1 – dated 1st June 2015 Implementation 01/07/2015Documento9 páginasPCN ISI/ Appendix Z1 Issue 1 – dated 1st June 2015 Implementation 01/07/2015Brandon EricksonAún no hay calificaciones
- Ryj Hvac CatalogDocumento100 páginasRyj Hvac CataloganthonptbgAún no hay calificaciones
- AEL02237 SDS - DISPERBYK-2200 - US - enDocumento10 páginasAEL02237 SDS - DISPERBYK-2200 - US - enRıdvan SürmeliAún no hay calificaciones
- The Ultimate GHS Hazard Classification Guide - ERA Software SolutionsDocumento32 páginasThe Ultimate GHS Hazard Classification Guide - ERA Software SolutionsDina AzizAún no hay calificaciones
- Deeper Neet DCT - ChemistryDocumento8 páginasDeeper Neet DCT - Chemistryhbhaiya643Aún no hay calificaciones
- ảnh hưởng của stress mặn đối với lúaDocumento18 páginasảnh hưởng của stress mặn đối với lúa20.Nguyễn Hà MyAún no hay calificaciones
- TT 2023 Sem 1 Odd - Class 4e (Updated 311222)Documento1 páginaTT 2023 Sem 1 Odd - Class 4e (Updated 311222)Lim Zhe Xian (Bukitviewss)Aún no hay calificaciones
- 41 Symplocos Racemosa - MonographDocumento9 páginas41 Symplocos Racemosa - MonographMSKCAún no hay calificaciones
- Student Packet 11:3Documento54 páginasStudent Packet 11:3faisalalqadahibi100Aún no hay calificaciones
- TS - X Chemistry All DCEB Papers Chapter Wise Academic Standard Wise Prefinal - I & 2 QuestionsDocumento40 páginasTS - X Chemistry All DCEB Papers Chapter Wise Academic Standard Wise Prefinal - I & 2 Questionsc18180707Aún no hay calificaciones
- TMP0025U Products Accessories GuideDocumento40 páginasTMP0025U Products Accessories GuideMajdi BelguithAún no hay calificaciones
- Food Capture, Appetite, Digestion Rate and Efficiency in Hatchling and Juvenile Crocodylus Porosus.Documento24 páginasFood Capture, Appetite, Digestion Rate and Efficiency in Hatchling and Juvenile Crocodylus Porosus.Juan Pablo PalacioAún no hay calificaciones
- Fruit Enzymes LabDocumento10 páginasFruit Enzymes Labapi-340117487Aún no hay calificaciones
- 5052 Aluminum Sheet SuppliersDocumento13 páginas5052 Aluminum Sheet Supplierssanghvi overseas incAún no hay calificaciones
- Ferrx 5000 Magnetic SeparatorDocumento6 páginasFerrx 5000 Magnetic SeparatorleontoledoAún no hay calificaciones