Documentos de Académico
Documentos de Profesional
Documentos de Cultura
Lab 3 Kinetics
Cargado por
Michelle SamayoaDescripción original:
Derechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Lab 3 Kinetics
Cargado por
Michelle SamayoaCopyright:
Formatos disponibles
Marcie Samayoa Period 1 AP Chemistry
Determining the Rate of a Reaction
Purpose:
February 13, 2010
Investigate how the rate of reaction can be measured and how reaction conditions affect reaction rates. Procedure: Part 1. Find the Volume of One Drop of Solution 1. 2. 3. 4. Fill the pipet with deionized water. Record the mass of a small beaker. Put five drops of water in the beaker and find the total mass. Record data. Add an additional five drops of water into the beaker, and again determine the mass. Record data. 5. Put in an additional five more drops and find the mass. Record data. Part 2. Determine the Reaction Rate and Calculate the Rate Law 1. Obtain six pipets and label the pipets KI, H2O, HCl, Starch, Na2S2O3, and KBrO3. 2. Using the table given as a guide, fill each numbered well in the first reaction strip. Mix the solution in each well with a toothpick. 3. To wells 1-9 in the second reaction strip, add 2 drops of 0.040 M KBrO3. 4. Record the time when the solution in each cell turns blue. 5. When all the cells have turned blue, take the temperature of one of the reaction solutions. Record this temperature for all the reactions. 6. Repeat steps 4-11 for the combinations that cover experiments 4 and 5 and experiments 6 and 7. Part 3. Determine the Activation Energy 1. Prepare a warm water bath of about 40C. 2. Fill the first six wells in the reaction strip with the appropriate number of drops of the reagent listed. Mix solutions with a toothpick. 3. Place the reaction strip in the water bath with KBrO3 pipet. 4. Measure the temperature of the water bath with a thermometer and record the values. 5. Add 2 drops of KBrO3 and record time when the first blue color appears. 6. Repeat steps 7-9 for the reaction solutions in wells #2 and #3.
7. Add ice cubes and water to create a cold temperature water bath. 8. Place the reaction strip and KBrO3 pipet into the cold temperature water bath. 9. Measure the temperature of the water bath with a thermometer and record values. 10. Repeat steps 7-9 for wells #4, #5, and #6. Record the time for each reaction. Part 4. Observe the Effect of a Catalyst on the Rate. Repeat the procedure in Part 2 for Experiment 1 only and add 1 drop of 0.1 M cupric nitrate solution. Cu(NO3)2, and 3 drops of distilled water to the mixture. Fill only the first reaction wells. Record the reaction times.
Data: Part 1. Data Table. Find the Volume of One Drop of Solution Mass of empty beaker (a) Trial 1 Mass of beaker plus 5 drops of water (b) Mass of first 5 drops of water (b) - (a) Average mass of 1 drop of water Trial 2 Mass of beaker plus 10 drops of water (c) Mass of second 5 drops of water (c) - (b) Average mass of 1 drop of water Trial 3 Mass of beaker plus 15 drops of water (d) Mass of third 5 drops of water (d) (c) Average mass of 1 drop of water Average mass of 1 drop of water (Trial 1-3) 28.56 28.78 0.220 0.044 29 0.220 0.044 29.2 0.230 0.046 .0446 mL
Part 2. Data Table. Determine the Reaction Rate and Calculate the Rate Law. Time, seconds Temp C Experiment No. 1 2 3 4 5 6 7 8 Trial 1 22 17.6 8.5 19.3 13.7 7.6 4.1 6.1 Trial 2 24.1 17.4 8.7 22.7 11.3 8.4 4.3 4.9 Average 23.05 17.5 8.6 21 12.5 8 4.2 5.5 24.6C 24.6C 24.6C 24.6C 24.6C 24.6C 24.6C 24.6C 6.0310-7 7.9410-7 1.6110-6 6.6210-7 1.1110-6 1.7410-6 3.3110-6 2.5310-6 .0010 .0020 .0030 .0010 .0010 .0010 .0010 .0015 .0067 .0067 .0067 .0133 .0200 .0067 .0067 .0100 .0167 .0167 .0167 .0167 .0167 .0333 .0500 .0250 Reaction Rate M/s Initial Concentrations, M [I-] [BrO3-] [H+]
Part 3. Data Table. Determine the Activation Energy.
Time of Reaction, seconds Approxi mate Temperat ure, C Measured Temperature C Temperature ,K 1/Temperature K-1 Trial 1 Trial 2 Average Time Rate of Reaction M/s Rate Constant, k (with units) 126 M-3s-1 247 M-3s-1 264 M-3s-1 706 M-3s-1 Natu ral Log k
0C 10 C 20 C 40 C
0.7 C 10..1 C 21.5 C 38.5 C
273.7 283.1 294.5 311.5
3.6510 3.5310 3.3910 3.2110
-3 -3 -3 -3
1:02:00 0:30:01 0:26:02 0:10:09
0:56:0 0:30:02 0:30:01 0:10:01
0:59:0 0:30:15 0:28:15 0:10:50
2.3510 4.6110 4.9410 1.3210
-7 -7 -7 -7
4.83 5.50 5.57 6.55
Part 4. Data Table. Observe the Effect of a Catalyst on the Rate Reaction Time, seconds Trial 1 Trial 2 17.5 seconds 17.9 seconds
Calculations: Find the Rate Constant Experiment Value of K Average Value Conclusion From the results of the lab, it can be concluded that the reaction conditions affect reaction rates. An experiment was conducted at specific concentrations of each of the reactants and then used to measure the reaction rate. Then, the concentration of one reactant is changed and thus shows how the reaction rate changes. It was repeated for each reactant and it allowed the calculation of the order of each reactant. Once the orders were known, which resulted in the rate law: Rate = k [I-] [BrO3-] [H+]2, the value of the rate constant was calculated in which averaged out to be 232M-3s-1. By using a separate experiment, the activation energy was also determined by measuring the time it took for a reaction to take place at different temperatures. Data was recorded and used to find the natural log of k and temperature in the Kelvin scale. The points were graphed and the slope of the best fit line was found and put into an equation which resulted in the activation energy of 27.8 kJ. Catalysts also affect reaction rates. Catalysts (Copper II Nitrate) was added in the same conditions of Experiment 1 and resulted in a faster reaction of 17.5 and 17.9 seconds (Trial 1 and 2, respectively) than the 23 seconds recorded in the original experiment 1. Theory Analysis The rate law for the lab resulted to be: Rate = k [I-] [BrO3-] [H+]2. The rate law was found to see which of the reactants will have an impact on a reaction rate. For example, when Experiment 1 and Experiment 7 are compared, it is obvious that Experiment 7 reacts a lot faster than Experiment 1. This is because Experiment 7 had higher volumes of HCl in the solution and 1
322 M-3s-1
2
212 M-3s-1
3
287 M-3s-1
4
178 M-3s-1 232 M-3s-1
5
199 M-3s-1
6
234 M-3s-1
7
197 M-3s-1
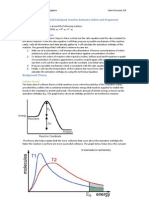
since [Cl-] is included in the rate law it will thus affect the reaction rate. Experiment 1 also had HCl but less of it due to the fact that water took in some of its place and since water is not in the rate law, the addition of water did not affect the reaction rate and with less volumes of the reactants that appear in the rate law, it does not proceed faster than Experiment 7. In Part 3. Data Table. Determine the Activation Energy it can be seen that the higher the temperature, the faster the reaction will take place. This is due to the fact that an increase of temperature increases the chance of sufficient energy for a reaction and chance of collision at a reactive site, and vice versa. Both are factors that affect the rate of chemical reactions. Lastly, catalysts also speed up the rate of chemical reactions. Without a catalyst, experiment 1 takes an average of about 23.05 seconds to react. When Copper II Nitrate (catalyst) is added to Experiment 1, the reaction speeds up by taking an average of about 17.7 seconds to react. This occurs because the catalyst changed the mechanism of the reaction providing an alternate pathway that decreases the activation energy and thus making the reaction go faster. Questions 1. The higher the concentration, the greater the chance of collision and the faster the reaction rate. If the concentration decreases, there is a less chance of collision and therefore the slower the reaction rate. 2. Determine the rate law by comparing two trials where the concentration of only one reactant changes. Find the quotient of the rate of reaction and the quotient of the concentrations of the changing chemical and solve for the exponent that would come with the concentrations. Use logarithms if needed to solve for the exponent and the exponent would determine the order it is in the rate law. This has to be done for every chemical involved in the reaction, individually. 3. It is reasonable since there was a few factors that could have affected the lab data including air bubbles, the starch solution (not completely dissolved), and dirty wells. 4. As the temperature increases, there is a greater chance of sufficient energy for the reaction and chance of collision at the reactive site so therefore faster reaction rates. As the temperature decreases, there is not sufficient energy for the reaction and therefore less chance of collision at the reactive site so therefore slower reaction rates. 5. To find the activation energy, the slope of the best fit line of ln k vs. (1/Temperature K-1) needs to be determined. Once it is found, it can be put in the equation: slope = -Ea/R. Afterwards, solve for Ea and that would equal to the activation energy. 6. The reaction rate is defined as how fast a reaction takes place. Unlike the reaction rate, the specific constant rate is an experimentally determined constant, which is different for different reactions and changes only with temperature 7. A catalyst is a substance that speeds up the rate of a reaction without being consumed in the reaction. When a catalyst is added to a reaction, it provides an alternate pathway that decreases the activation energy and thus making the reaction go faster. 8. Skip.
9. My data was not completely accurate since there was a 16% difference between rates of reaction between Experiment 8 and the data. 10. By using cleaner wells, it reduces the chances of the chemicals reacting with other unwanted chemicals in the dirty wells. Air bubbles could have changed the amount of concentration that was needed by using less of the concentration than of the ones that was actually needed to put in the experiments. This could have affected the data dramatically since only a little amount of volume of chemicals was used in the lab so less air bubbles could improve the results of the lab. The starch not dissolving well in water could have given different concentrations then the one listed in the lab affecting the lab dramatically also. If the starch was completely dissolved, it could have produced better lab results.
También podría gustarte
- AP Chemistry - Kinetics of A Reaction LabDocumento8 páginasAP Chemistry - Kinetics of A Reaction LabJonathan Chen50% (2)
- SAMPLE MCQuestions ByTopicsDocumento45 páginasSAMPLE MCQuestions ByTopicsVeeru ManikantaAún no hay calificaciones
- Audi A4-7Documento532 páginasAudi A4-7Anonymous QRVqOsa5Aún no hay calificaciones
- There Will Come Soft RainsDocumento8 páginasThere Will Come Soft RainsEng ProfAún no hay calificaciones
- Governance Operating Model: Structure Oversight Responsibilities Talent and Culture Infrastructu REDocumento6 páginasGovernance Operating Model: Structure Oversight Responsibilities Talent and Culture Infrastructu REBob SolísAún no hay calificaciones
- P3 Past Papers Model AnswersDocumento211 páginasP3 Past Papers Model AnswersEyad UsamaAún no hay calificaciones
- Code of Ethics For Civil Engineers PiceDocumento3 páginasCode of Ethics For Civil Engineers PiceEdwin Ramos Policarpio100% (3)
- Chem 26.1 Formal Report Experiment 3 Iodine Clock ReactionDocumento5 páginasChem 26.1 Formal Report Experiment 3 Iodine Clock ReactionromiYAY71% (7)
- Reaction KineticsDocumento7 páginasReaction Kineticsjathan160% (1)
- Kinetics of Reaction: The Iodine Clock RotationDocumento13 páginasKinetics of Reaction: The Iodine Clock RotationDavid Lemuel del PradoAún no hay calificaciones
- Effect of Temperature On The Reaction RateDocumento5 páginasEffect of Temperature On The Reaction RateChristy Joy RetanalAún no hay calificaciones
- National Interest Waiver Software EngineerDocumento15 páginasNational Interest Waiver Software EngineerFaha JavedAún no hay calificaciones
- Expt 6 - Chemical KineticsDocumento5 páginasExpt 6 - Chemical KineticsNeil Tangara100% (2)
- Practice Makes Perfect in Chemistry: Kinetics and Equilibrium with AnswersDe EverandPractice Makes Perfect in Chemistry: Kinetics and Equilibrium with AnswersAún no hay calificaciones
- Expt. #3 - FRDocumento9 páginasExpt. #3 - FRClarice Mae DacasinAún no hay calificaciones
- Experiment 6 FinalDocumento13 páginasExperiment 6 FinalFroileth Pulido100% (1)
- Sample Kinetics ExperimentDocumento7 páginasSample Kinetics ExperimentVenus PondevidaAún no hay calificaciones
- Exp 4 Kinetics Order of ReactionDocumento8 páginasExp 4 Kinetics Order of ReactionNur Fadhilah0% (1)
- The Kinetic Study of The IodinationDocumento6 páginasThe Kinetic Study of The IodinationsamAún no hay calificaciones
- Lab Report-Exp 1Documento8 páginasLab Report-Exp 1abmarti1Aún no hay calificaciones
- Isothermal Batch ReactorDocumento10 páginasIsothermal Batch ReactorSaswiny Ritchie0% (2)
- Rate Factors LabDocumento4 páginasRate Factors LabAadilAún no hay calificaciones
- Rate ReactionDocumento10 páginasRate ReactionTsabit AlbananiAún no hay calificaciones
- Iodine Clock Kinetics Exp 9 Communication ReportDocumento2 páginasIodine Clock Kinetics Exp 9 Communication Reportapi-530290964Aún no hay calificaciones
- Experiment 1Documento5 páginasExperiment 1Joe CslAún no hay calificaciones
- AP Chemistry, Kinetics Lab ReportDocumento15 páginasAP Chemistry, Kinetics Lab ReportSebatHian Santiago60% (5)
- Kinetics Expt 4-2011Documento7 páginasKinetics Expt 4-2011Wilo JaraAún no hay calificaciones
- Reaction RateDocumento19 páginasReaction RateMuhd Hafiz NizamAún no hay calificaciones
- Reaction Kinetics-Ver 2Documento4 páginasReaction Kinetics-Ver 2Ivan Alberto NinaAún no hay calificaciones
- Chemical KineticsDocumento6 páginasChemical KineticsWiktoria KaczmarzykAún no hay calificaciones
- Experiment 2: Kinetics of The Reaction Between Permanganate and Oxalic AcidDocumento4 páginasExperiment 2: Kinetics of The Reaction Between Permanganate and Oxalic AcidMaryNicoleDatlanginAún no hay calificaciones
- Formal Written Lab Report - Experiment 1 - Group 1 (1st Year - Summer Sem)Documento7 páginasFormal Written Lab Report - Experiment 1 - Group 1 (1st Year - Summer Sem)Cheska BiolenaAún no hay calificaciones
- Formal Report in Analytical ChemistryDocumento5 páginasFormal Report in Analytical ChemistryJohn Rally Jr FilamorAún no hay calificaciones
- Liquid Phase Chemical Reactor FinalDocumento38 páginasLiquid Phase Chemical Reactor FinalToMemAún no hay calificaciones
- Exp 3 Kinetics - Factors Affecting Rates of ReactionDocumento6 páginasExp 3 Kinetics - Factors Affecting Rates of ReactionMuhammad Amirul AfifiAún no hay calificaciones
- Experiment: Factors Affecting Rate of ReactionsDocumento10 páginasExperiment: Factors Affecting Rate of ReactionsAnonymous BPFqriLDAún no hay calificaciones
- Kinetic ExampleDocumento6 páginasKinetic ExampleEqieyn JerrAún no hay calificaciones
- 112 Experiment 3Documento3 páginas112 Experiment 3Amal ..Aún no hay calificaciones
- Rates and Energetics Mastery BookletDocumento20 páginasRates and Energetics Mastery Bookletapi-422428700Aún no hay calificaciones
- Chemical Kinetics Methodology, RDRDocumento7 páginasChemical Kinetics Methodology, RDRKhayzel MelanoAún no hay calificaciones
- Physical Chemistry 1 (CHM 471) : Faculty of Applied Sciences Laboratory ReportDocumento10 páginasPhysical Chemistry 1 (CHM 471) : Faculty of Applied Sciences Laboratory ReportHusna Insyirah Bt SamadAún no hay calificaciones
- Iodination Lab Report1Documento5 páginasIodination Lab Report1Sherlock Wesley ConanAún no hay calificaciones
- Investigating The Effect of Temperature & PH On Enzyme ActivityDocumento3 páginasInvestigating The Effect of Temperature & PH On Enzyme Activityjuliakang03Aún no hay calificaciones
- C STR Kinetics 2012Documento12 páginasC STR Kinetics 2012JpojAún no hay calificaciones
- Kinetics of The Acid Catalysed Reaction Between Iodine and Propanone Final LolDocumento23 páginasKinetics of The Acid Catalysed Reaction Between Iodine and Propanone Final LolYann Perusset90% (10)
- Chapter 8 Chemical ReactionsDocumento15 páginasChapter 8 Chemical ReactionsAmmar RizwanAún no hay calificaciones
- Dordas, Rey A. - Activity 5 - Lab ExerciseDocumento3 páginasDordas, Rey A. - Activity 5 - Lab ExerciseRey DordasAún no hay calificaciones
- Lab Chem 3 :kinetics-Factor Affecting Rate of ReactionDocumento2 páginasLab Chem 3 :kinetics-Factor Affecting Rate of ReactionNadiah Matarsim60% (5)
- Kinetics: 6.1 Rates of ReactionDocumento20 páginasKinetics: 6.1 Rates of ReactionSeung Hee KimAún no hay calificaciones
- Chem A 13 Comp EnthalpyDocumento5 páginasChem A 13 Comp EnthalpyKrystela Cariza Ramos MercadoAún no hay calificaciones
- Chemical Kenetics - 17 Exp 3Documento4 páginasChemical Kenetics - 17 Exp 3Bobbi DoloirasAún no hay calificaciones
- Lab ReportpdfDocumento7 páginasLab ReportpdfStefano FochesattoAún no hay calificaciones
- 123Documento6 páginas123Julius Rafael Delprado DildigAún no hay calificaciones
- Experiment 4: Effect of Concentration and Temperature On Rate of Reaction (Dissappearing Cross)Documento24 páginasExperiment 4: Effect of Concentration and Temperature On Rate of Reaction (Dissappearing Cross)Malini RajeshAún no hay calificaciones
- Chemical Kinetics FRDocumento6 páginasChemical Kinetics FRJo FernandezAún no hay calificaciones
- The Indian SchoolDocumento21 páginasThe Indian SchoolSabreena BasheerAún no hay calificaciones
- Catalyzed Decomposition of Hydrogen PeroxideDocumento5 páginasCatalyzed Decomposition of Hydrogen PeroxideDennis WrinAún no hay calificaciones
- Iodination of Acetone KineticsDocumento4 páginasIodination of Acetone Kineticsinstrutech0% (2)
- Oxidation of Isopropanol by Chromium (Vi) ReportDocumento12 páginasOxidation of Isopropanol by Chromium (Vi) ReportGideonAún no hay calificaciones
- LR - KineticsDocumento9 páginasLR - Kineticsapi-357715756Aún no hay calificaciones
- Chem 17 Exp 3 RDR Chemical KineticsDocumento4 páginasChem 17 Exp 3 RDR Chemical KineticscrazypatrishAún no hay calificaciones
- Saturday X-Tra X-Sheet: 16 Rates of Reaction: Key ConceptsDocumento6 páginasSaturday X-Tra X-Sheet: 16 Rates of Reaction: Key ConceptsKingford MwesoAún no hay calificaciones
- M5A1 Hess's Law LabDocumento11 páginasM5A1 Hess's Law LabBryan HatchAún no hay calificaciones
- Ox Alic Acid KineticsDocumento10 páginasOx Alic Acid Kineticsmkhurram79Aún no hay calificaciones
- Practice Makes Perfect in Chemistry: Kinetics and EquilibriumDe EverandPractice Makes Perfect in Chemistry: Kinetics and EquilibriumAún no hay calificaciones
- Physico-Chemistry of Solid-Gas Interfaces: Concepts and Methodology for Gas Sensor DevelopmentDe EverandPhysico-Chemistry of Solid-Gas Interfaces: Concepts and Methodology for Gas Sensor DevelopmentAún no hay calificaciones
- Thermometric Titrimetry: International Series of Monographs in Analytical ChemistryDe EverandThermometric Titrimetry: International Series of Monographs in Analytical ChemistryAún no hay calificaciones
- 7 1 13Documento2 páginas7 1 13Michelle SamayoaAún no hay calificaciones
- Must Be A Well Written and Organized Essay) 1. Description: Explain Main Argument? What Is BeingDocumento2 páginasMust Be A Well Written and Organized Essay) 1. Description: Explain Main Argument? What Is BeingMichelle SamayoaAún no hay calificaciones
- Ali MohamedDocumento19 páginasAli MohamedMichelle SamayoaAún no hay calificaciones
- Is Gay Marriage RacistDocumento7 páginasIs Gay Marriage RacistMichelle SamayoaAún no hay calificaciones
- Midterm Study Guide 1Documento5 páginasMidterm Study Guide 1Michelle SamayoaAún no hay calificaciones
- Acids and Bases Review Packet - KEYDocumento11 páginasAcids and Bases Review Packet - KEYMichelle Samayoa100% (1)
- A Pun It 14 EquilibriumDocumento50 páginasA Pun It 14 EquilibriumMichelle SamayoaAún no hay calificaciones
- AP Calculus AB Exam, Number 6 2007Documento8 páginasAP Calculus AB Exam, Number 6 2007Michelle SamayoaAún no hay calificaciones
- AMS ANALITICA-AIRFLOW TSP-HVS BrochureDocumento1 páginaAMS ANALITICA-AIRFLOW TSP-HVS BrochureShady HellaAún no hay calificaciones
- BIOAVAILABILITY AND BIOEQUIVALANCE STUDIES Final - PPTX'Documento32 páginasBIOAVAILABILITY AND BIOEQUIVALANCE STUDIES Final - PPTX'Md TayfuzzamanAún no hay calificaciones
- IPA Smith Osborne21632Documento28 páginasIPA Smith Osborne21632johnrobertbilo.bertilloAún no hay calificaciones
- Job Satisfaction of Library Professionals in Maharashtra State, India Vs ASHA Job Satisfaction Scale: An Evaluative Study Dr. Suresh JangeDocumento16 páginasJob Satisfaction of Library Professionals in Maharashtra State, India Vs ASHA Job Satisfaction Scale: An Evaluative Study Dr. Suresh JangeNaveen KumarAún no hay calificaciones
- File RecordsDocumento161 páginasFile RecordsAtharva Thite100% (2)
- English Test For Grade 7 (Term 2)Documento6 páginasEnglish Test For Grade 7 (Term 2)UyenPhuonggAún no hay calificaciones
- Management PriniciplesDocumento87 páginasManagement Priniciplesbusyboy_spAún no hay calificaciones
- (EN 10348) - Steel For The Reinforcement of Concrete. Galvanized Reinforcing SteelDocumento24 páginas(EN 10348) - Steel For The Reinforcement of Concrete. Galvanized Reinforcing Steelbagusu_6Aún no hay calificaciones
- SCD Course List in Sem 2.2020 (FTF or Online) (Updated 02 July 2020)Documento2 páginasSCD Course List in Sem 2.2020 (FTF or Online) (Updated 02 July 2020)Nguyễn Hồng AnhAún no hay calificaciones
- RSW - F - 01 " ": Building UtilitiesDocumento4 páginasRSW - F - 01 " ": Building Utilities62296bucoAún no hay calificaciones
- MPI Unit 4Documento155 páginasMPI Unit 4Dishant RathiAún no hay calificaciones
- Amity School of Business:, Semester IV Research Methodology and Report Preparation Dr. Deepa KapoorDocumento23 páginasAmity School of Business:, Semester IV Research Methodology and Report Preparation Dr. Deepa KapoorMayank TayalAún no hay calificaciones
- Man Bni PNT XXX 105 Z015 I17 Dok 886160 03 000Documento36 páginasMan Bni PNT XXX 105 Z015 I17 Dok 886160 03 000Eozz JaorAún no hay calificaciones
- Cameron International Corporation: FORM 10-KDocumento31 páginasCameron International Corporation: FORM 10-KMehdi SoltaniAún no hay calificaciones
- Gas Compressor SizingDocumento1 páginaGas Compressor SizingNohemigdeliaLucenaAún no hay calificaciones
- Use of The Internet in EducationDocumento23 páginasUse of The Internet in EducationAlbert BelirAún no hay calificaciones
- Engineering Ethics in Practice ShorterDocumento79 páginasEngineering Ethics in Practice ShorterPrashanta NaikAún no hay calificaciones
- Bring Your Gear 2010: Safely, Easily and in StyleDocumento76 páginasBring Your Gear 2010: Safely, Easily and in StyleAkoumpakoula TampaoulatoumpaAún no hay calificaciones
- Week 3 Lab Arado, Patrick James M.Documento2 páginasWeek 3 Lab Arado, Patrick James M.Jeffry AradoAún no hay calificaciones
- Chapter 2 Short-Term SchedulingDocumento49 páginasChapter 2 Short-Term SchedulingBOUAZIZ LINAAún no hay calificaciones
- Resume - James MathewsDocumento2 páginasResume - James Mathewsapi-610738092Aún no hay calificaciones
- Kübler 5800-5820 - enDocumento5 páginasKübler 5800-5820 - enpomsarexnbAún no hay calificaciones
- ISO Position ToleranceDocumento15 páginasISO Position ToleranceНиколай КалугинAún no hay calificaciones