Documentos de Académico
Documentos de Profesional
Documentos de Cultura
Chapter 12
Cargado por
wasimiitgnDerechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Chapter 12
Cargado por
wasimiitgnCopyright:
Formatos disponibles
Chapter 12: MECHANICAL SEPARATION
CHAPTER
TWELVE
MECHANICAL SEPARATION
12.2 Sedimentation
The Meaning of Success To earn the respect of intelligent people and to win the affection of children; To appreciate the beauty in nature and all that surrounds us; To seek out and nurture the best in others; To give the gift of yourself to others without the slightest through of return, for it is in giving that we receive; To have accomplished a task, whether it be saving a lost soul, healing a sick child, writing a book, or risking your life for a friend; To have hope even in times of despair, for as long as you have hope, you have life; To love and to be loved; To be understood and to understand; To know that even one life has breathed easier because you have lived; This is the meaning of success. ANONYMOUS
12.2. SEDIMENTATION
Keywords: Sedimentation, settling velocity, settling rate
12.2.1. Object
The aim of this experiment is to determine the rate at which the rate of settling of solid is a minimum, the settling velocity at this concentration and the amount of water rejected from the slurry between this concentration and that of the product, i.e. the amount that is flowing upwards at the limiting level.
12.2.2. Theory
Sedimentation is the partial separation or concentration of suspended solid particles from a liquid by gravity settling. This field may be divided into the functional operations of thickening and clarification. The primary purpose of thickening is to increase the concentration of suspended solids in a feed stream, while that of clarification is to remove a relatively small quantity of suspended particles and procedure a clear effluent.
The factors effecting sedimentation are: The nature of particles: size distribution, shape, specific gravity, chemical and mineralogical properties, etc.
Concentration effects Type of pretreatment: chemical conditioning, flocculation, heating, cooling, etc. Type of containing vessel: size, shape, wall effects, etc.
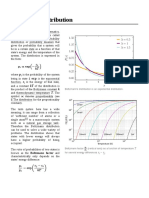
The Talmage-Fitch method which is used to construct the settling velocity versus concentration graphs, enables the construction of graph from a single batch sedimentation experiment.
When solid particles deposit on the bottom of the column, they displace some water and cause it to move upwards. This upflow carries some of the suspended particles with itself. This upthrust is indicated by VLU, where the settling velocity of particles is indicated by VL.
Chapter 12: MECHANICAL SEPARATION
If Co and Zo represents the initial concentration and height of the suspended solids in a batch settling test then total weight of the solids in the slurry is CoZoS where S is the cross-sectional area of batch settlement cylinder.
To treat the batch settling data, the height of the interphase as a function of time is plotted. From this plot, the value of VL is the slope of the curve at =L since the tangent with the curve at intersects the ordinate at a height of interphase Zi and the slope of the line is:
VL = or
Zi Z L L
Z i = Z L + L VL
A mass balance on solids yields C L Z i S = C 0 Z 0S or C L Zi = C0 Z0
therefore Zi is the height which slurry would occupy if all the solids present were at concentration CL, CL is the minimum concentration which boundary layers interfere.
12.2.3. Apparatus
The apparatus of this experiment consists of a long glass cylinder, CaCO3 slurry, length measuring device, stopwatch.
12.2.4. Experimental Procedure
1. 2.
Measure and record the height and diameter of the glass cylinder. Prepare CaCO3 solution in water at a certain concentration and pour into the cylinder at a certain height. Measure the length of the interface at certain time intervals until all the CaCO3 is settled down to the bottom of the glass cylinder.
3.
12.2.5. Report Objectives
1.
Plot a curve of the height of the interphase against time and obtain the curve of settling rate from this curve.
2.
Calculate the minimum settling rate, velocity and amount of rejected water.
12.2.6. References
1. Coulson, J.M., J.F. Richardson, Chemical Engineering Unit Operations, Pergamon Press, New York, 1962. 2. Perry, R.H., D. Green, Perrys Chemical Engineers Handbook, 6th edition, McGraw-Hill, 1988.
También podría gustarte
- Rabindranath TagoreDocumento45 páginasRabindranath Tagorewasimiitgn100% (1)
- Permutations and CombinationsDocumento1 páginaPermutations and CombinationswasimiitgnAún no hay calificaciones
- Printing & PackagingDocumento28 páginasPrinting & Packagingwasimiitgn0% (1)
- Cilt Paper 1Documento3 páginasCilt Paper 1wasimiitgnAún no hay calificaciones
- CILT - Research OutlineDocumento3 páginasCILT - Research OutlinewasimiitgnAún no hay calificaciones
- Viscosity of PolymersDocumento12 páginasViscosity of PolymerssevanthAún no hay calificaciones
- Fragmentation Test Method For Adhesion Analysis of Coatings in Situ in A MicroscopeDocumento4 páginasFragmentation Test Method For Adhesion Analysis of Coatings in Situ in A MicroscopewasimiitgnAún no hay calificaciones
- Agitator DesignDocumento6 páginasAgitator Designप्रमोद रणपिसेAún no hay calificaciones
- Report Rohit 12th JulyDocumento9 páginasReport Rohit 12th JulywasimiitgnAún no hay calificaciones
- Report Piyush 12th JulyDocumento12 páginasReport Piyush 12th JulywasimiitgnAún no hay calificaciones
- Applica Ation Fo or Add D/Drop O of Cour Rses: Course Es To A DDDocumento1 páginaApplica Ation Fo or Add D/Drop O of Cour Rses: Course Es To A DDwasimiitgnAún no hay calificaciones
- Writing Effective Statement of InterestDocumento4 páginasWriting Effective Statement of InterestBhoomika MansharamaniAún no hay calificaciones
- 2008 Urea Production Reduces Greenhouse GasDocumento8 páginas2008 Urea Production Reduces Greenhouse GaswasimiitgnAún no hay calificaciones
- Ammonia and Urea ProductionDocumento10 páginasAmmonia and Urea Productionwaheed_bhattiAún no hay calificaciones
- Roshan ResumeDocumento3 páginasRoshan ResumeRicha MalhotraAún no hay calificaciones
- 1355233729-MyntraShoppingFestival FAQs VF 12decDocumento5 páginas1355233729-MyntraShoppingFestival FAQs VF 12decwasimiitgnAún no hay calificaciones
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDe EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeCalificación: 4 de 5 estrellas4/5 (5784)
- The Yellow House: A Memoir (2019 National Book Award Winner)De EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Calificación: 4 de 5 estrellas4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDe EverandNever Split the Difference: Negotiating As If Your Life Depended On ItCalificación: 4.5 de 5 estrellas4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeDe EverandShoe Dog: A Memoir by the Creator of NikeCalificación: 4.5 de 5 estrellas4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDe EverandThe Emperor of All Maladies: A Biography of CancerCalificación: 4.5 de 5 estrellas4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDe EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceCalificación: 4 de 5 estrellas4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingDe EverandThe Little Book of Hygge: Danish Secrets to Happy LivingCalificación: 3.5 de 5 estrellas3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnDe EverandTeam of Rivals: The Political Genius of Abraham LincolnCalificación: 4.5 de 5 estrellas4.5/5 (234)
- Grit: The Power of Passion and PerseveranceDe EverandGrit: The Power of Passion and PerseveranceCalificación: 4 de 5 estrellas4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDe EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaCalificación: 4.5 de 5 estrellas4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDe EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryCalificación: 3.5 de 5 estrellas3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealDe EverandOn Fire: The (Burning) Case for a Green New DealCalificación: 4 de 5 estrellas4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDe EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureCalificación: 4.5 de 5 estrellas4.5/5 (474)
- Rise of ISIS: A Threat We Can't IgnoreDe EverandRise of ISIS: A Threat We Can't IgnoreCalificación: 3.5 de 5 estrellas3.5/5 (137)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDe EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersCalificación: 4.5 de 5 estrellas4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaDe EverandThe Unwinding: An Inner History of the New AmericaCalificación: 4 de 5 estrellas4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDe EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyCalificación: 3.5 de 5 estrellas3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDe EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreCalificación: 4 de 5 estrellas4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)De EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Calificación: 4.5 de 5 estrellas4.5/5 (119)
- The Perks of Being a WallflowerDe EverandThe Perks of Being a WallflowerCalificación: 4.5 de 5 estrellas4.5/5 (2099)
- Her Body and Other Parties: StoriesDe EverandHer Body and Other Parties: StoriesCalificación: 4 de 5 estrellas4/5 (821)
- MHH Gaussian Point-Reflection-New StepwiseDocumento18 páginasMHH Gaussian Point-Reflection-New StepwisesaddamAún no hay calificaciones
- Calibrating Bourdon Pressure GaugeDocumento9 páginasCalibrating Bourdon Pressure GaugeMuizzuddin Saleh100% (4)
- Film Characteristic Curves RTDocumento3 páginasFilm Characteristic Curves RTMomo ItachiAún no hay calificaciones
- Graphical ExerciseDocumento11 páginasGraphical ExerciseOshane So-confidential SawyersAún no hay calificaciones
- JAR Part 66 Exam Mod 02Documento92 páginasJAR Part 66 Exam Mod 02Shreyas PingeAún no hay calificaciones
- Magnetorheological Fluids As A Prospective Component of Composite ArmoursDocumento5 páginasMagnetorheological Fluids As A Prospective Component of Composite ArmoursSuwerenAún no hay calificaciones
- Oxidation-Reduction Write UpDocumento4 páginasOxidation-Reduction Write Upre5teAún no hay calificaciones
- S09 TTT DiagramDocumento6 páginasS09 TTT Diagrampraba_343Aún no hay calificaciones
- Introduction To Fission and FusionDocumento19 páginasIntroduction To Fission and FusionZubair Hassan100% (1)
- PVM Training Presentation Session 1 - 2014 SecureDocumento40 páginasPVM Training Presentation Session 1 - 2014 Securediaccessltd_17172961Aún no hay calificaciones
- Chapter 1Documento16 páginasChapter 1aregawi weleabezgiAún no hay calificaciones
- Theory of Vibration 5th Edition BookDocumento2 páginasTheory of Vibration 5th Edition BookSaad Afzal0% (1)
- Process of CommunicationDocumento11 páginasProcess of CommunicationEdhel CabalquintoAún no hay calificaciones
- Transformasi Linier-14Documento72 páginasTransformasi Linier-14Rizqie Puspita MayasariAún no hay calificaciones
- Montecarlo in AerodynamicsDocumento32 páginasMontecarlo in AerodynamicsVíctor QuezadaAún no hay calificaciones
- Boltzmann DistributionDocumento7 páginasBoltzmann Distributionhobson616Aún no hay calificaciones
- Incho11 QP Previous Year Question Papers of Indian National Chemistry Olympiad (INChO)Documento32 páginasIncho11 QP Previous Year Question Papers of Indian National Chemistry Olympiad (INChO)Akshay PandeyAún no hay calificaciones
- Introduction To Spectrochemical MethodsDocumento25 páginasIntroduction To Spectrochemical MethodsSİNEM GÜVENAún no hay calificaciones
- A Kinetic Equilibrium Study of A Triiodide Concentration Maximum Formed by The Persulfate Iodide ReactionDocumento3 páginasA Kinetic Equilibrium Study of A Triiodide Concentration Maximum Formed by The Persulfate Iodide ReactionArielDeCandia0% (1)
- TLWDocumento39 páginasTLWCandida DhasonAún no hay calificaciones
- Answers: Exercise 1.1Documento2 páginasAnswers: Exercise 1.1MazlinAún no hay calificaciones
- Introduction to High Voltage EngineeringDocumento30 páginasIntroduction to High Voltage Engineeringmuhammad saeedAún no hay calificaciones
- Sandstone Petrology and Provenance of The Neoproterozoic Voltaian Group in The Southeastern Voltaian Basin, GhanaDocumento16 páginasSandstone Petrology and Provenance of The Neoproterozoic Voltaian Group in The Southeastern Voltaian Basin, GhanaGrace NorteyAún no hay calificaciones
- KC22403 Lect1 2022Documento18 páginasKC22403 Lect1 2022NURUL YAHSIFAH SYQELLA BINTI YAHYA BK21110100Aún no hay calificaciones
- Electrodeposition of Nanocrystalline Zn-Ni Coatings With Single Gamma Phase From An Alkaline BathDocumento10 páginasElectrodeposition of Nanocrystalline Zn-Ni Coatings With Single Gamma Phase From An Alkaline BathIcanIbrahimAún no hay calificaciones
- Del in Cylindrical and Spherical CoordinatesDocumento2 páginasDel in Cylindrical and Spherical CoordinatesNoushad Bin JamalAún no hay calificaciones
- Chaptwer 3. Fluid Mechanics 1 (Section-I) Fluid Kinematics. P.EDocumento41 páginasChaptwer 3. Fluid Mechanics 1 (Section-I) Fluid Kinematics. P.EKennie RajAún no hay calificaciones
- Production of Fire-Clay Refractory Bricks From Local MaterialsDocumento7 páginasProduction of Fire-Clay Refractory Bricks From Local MaterialsFireboy NanthaAún no hay calificaciones
- SHAPES AND BOND ANGLES OF SIMPLE MOLECULESDocumento78 páginasSHAPES AND BOND ANGLES OF SIMPLE MOLECULESKareem MckenzieAún no hay calificaciones
- Nikhil MTP ReportDocumento19 páginasNikhil MTP ReportNikhilAún no hay calificaciones