Documentos de Académico
Documentos de Profesional
Documentos de Cultura
Melting Ice To Find The Value of The Latent Heat Capacity of Fusion of Ice
Cargado por
Marc WierzbitzkiTítulo original
Derechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Melting Ice To Find The Value of The Latent Heat Capacity of Fusion of Ice
Cargado por
Marc WierzbitzkiCopyright:
Formatos disponibles
Marc Wierzbitzki
Physics HL
20 January 2011
Melting ice to find the value of the latent heat capacity of fusion of ice
Aim: With the help of this experiment, we wanted to find the value of the latent heat capacity of fusion (Lfus) of water. Construction:
Method: After setting everything up as shown in the illustration above, reset the mass balance to 0 so that the mass of the beaker and the thermometer will be ignored, because only the mass of the ice and water matter. Start by filling the water into the big beaker and note down the temperature and mass. After doing that, also give in the ice and wait until it is completely melted. Note down the additional mass and the final temperature. Results: MI (g)
0.2g
MW(g)
0.2g
T1(I) (C)
1C
T2(W) (C)
1C
T2(C)
1C
MI TW (gK)
11%
MW TW (gK)
11%
E (J)
30%
21.30 45.90 50.00 19.30 30.50 41.97 77.21 20.08 07.25 37.25 46.16 04.50 15.20 54.20 68.60
150.00 112.00 220.00 164.10 189.00 133.87 133.38 100.00 100.00 100.00 100.00 050.45 050.30 163.70 163.50
0 0 0 0 0 0 0 0 0 0 0 0 0 0 0
53 40 56 65 34 49 44 45 48 42 52 57 60 65 67
40.0 13.0 36.0 52.0 23.0 18.5 07.5 25.5 40.0 17.0 16.0 46.0 35.0 33.0 29.0
0852 0597 1800 1004 0702 0776 0579 0512 0290 0633 0739 0207 0532 1785 1989
-1950 -3024 -4400 -2133 -2079 -4083 -4868 -1950 -0800 -2500 -3600 -0555 -1258 -5238 -6213
04597 10163 10886 04730 05768 13845 17959 06021 02135 07816 11981 01457 03038 14458 17684
Error analysis: The smallest error can be found in the masses of water and ice, as the mass balances we used were quite accurate. Comparing them to each other, the biggest deviation we noticed was 0.2g. The thermometers all displayed the same temperature, but as they were not digital, we could only read the results with an error of 1C, whereas a digital thermometer would have been more accurate. Using those values, we calculated that the error for MI TW has to be 11% and for E30%. Those two errors are quite big (as you can see in the graph below), which will make it harder for us to decide whether our final result is right or not.
Marc Wierzbitzki
Physics HL
20 January 2011
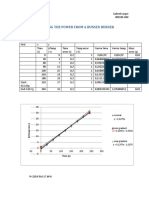
Graph:
Lfus
25000
20000
-(CW(MITI +MWTW )
15000
10000
5000
y = 147.65x + 1200.3 y = 245.15x + 19.706 y = 305.37x - 293.13 0 0 10 20 30 40 MI 50 60 70 80 90
The above graph gives a mean value of Lfus=245.15Jg-1. The minimum value is equal to 147.65 Jg-1, whereas the maximum value is 305.37 Jg-1. Therefore, we can compute an error to the mean value of 97.5 Jg-1. Looking at the result that we expected (L fus=334Jg-1), our result still fits into the error range. But on the other hand, the experiment is still not very conclusive, since the error is about 40% of the mean value and the range it gives us is simply too big.
The problem with this experiment was probably the amount of energy that was lost to the surroundings. This happened at two situations throughout the experiment and therefore the total error added up to 30%. Firstly, we assumed that the ice was constantly at 0C, which is not true. While doing the
Marc Wierzbitzki
Physics HL
20 January 2011
experiment, you could definitely see that more and more ice melted, it was wet as we did not put it back into a refrigerator after we had used it. Also, since the water was very hot, it constantly loosed mass through evaporation, which we did not capture. This affected the value of E (shown in the last column in the table). Improvements to this could lower the error for this value. Possible ways to improve the experiment: Looking at the problems that I described above, quite many improvements can be suggested. Firstly, since this is the most significant error source, we have to minimize the energy loss to surroundings. To do so, we should not use a beaker anymore but utilize a thermos flask, which would eliminate the effect of the temperature surroundings. Furthermore, we should also think about closing the flask entirely so that no water can evaporate and disappear, but it would be trapped within the flask. Also, it has to be ensured that the ice stays at the same temperature throughout the whole experiment, otherwise no conclusion can be drawn. To make this possible, the ice should be stored in a refrigerator until it is needed, just like the water should be boiled for each new repetition. To reduce all other errors as much as possible, a digital thermometer and mass balance should be used. Those two devices will reduce the errors in the mass and temperature readings since they are more accurate and no mistakes can be made when reading off the corresponding values. Redoing the experiment with the improved set-up will hopefully reduce the errors that have been mentioned above. Since we tried to eliminate the energy loss to surroundings, the results in general should be more accurate and closer to the actual value of 334Jg-1.
También podría gustarte
- The Ultimate ECAA Guide: Economics Admissions Assessment. Latest specification with 300+ practice questions with fully worked solutions, time saving techniques, score boosting strategies, and formula sheets.De EverandThe Ultimate ECAA Guide: Economics Admissions Assessment. Latest specification with 300+ practice questions with fully worked solutions, time saving techniques, score boosting strategies, and formula sheets.Aún no hay calificaciones
- Chemistry Higher Level Paper 1 Paper 2: Chemistry: Grade 11 End of Term Exam NameDocumento17 páginasChemistry Higher Level Paper 1 Paper 2: Chemistry: Grade 11 End of Term Exam NameJerry LouAún no hay calificaciones
- Instructions For Laboratory ReportsDocumento2 páginasInstructions For Laboratory ReportsCJAún no hay calificaciones
- Chemical Reaction Types PDFDocumento2 páginasChemical Reaction Types PDFSrinivasa Reddy Tummapudi100% (1)
- SMO Algebra IDocumento18 páginasSMO Algebra IJmex MkAún no hay calificaciones
- Physics Practical ExaminationDocumento1 páginaPhysics Practical ExaminationMohammed Bin SuhartoAún no hay calificaciones
- Pendulum WorksheetDocumento2 páginasPendulum Worksheetdr lailaAún no hay calificaciones
- Helicopter Lab 1Documento2 páginasHelicopter Lab 1Charina De la CruzAún no hay calificaciones
- Vieta's Formula - Brilliant Math & Science WikiDocumento9 páginasVieta's Formula - Brilliant Math & Science Wikiasax57Aún no hay calificaciones
- Math EE IBDocumento13 páginasMath EE IBCalc girl ReddingtonAún no hay calificaciones
- Olympiad Books Ambitious ListDocumento7 páginasOlympiad Books Ambitious ListJosé ParadaAún no hay calificaciones
- Physics EEDocumento4 páginasPhysics EEEjaz AhmadAún no hay calificaciones
- Wang Xiaomai Wheat Extended EssayDocumento33 páginasWang Xiaomai Wheat Extended Essay王小麦Aún no hay calificaciones
- AlgebraDocumento8 páginasAlgebraCambridgeSchool NoidaAún no hay calificaciones
- Physics Practical HandbookDocumento20 páginasPhysics Practical HandbookMOHAMED SHAMIR BIN TAJUDEENAún no hay calificaciones
- Stars and Bars Introduction To Competition MathDocumento36 páginasStars and Bars Introduction To Competition MathSatyam Kumar MalAún no hay calificaciones
- Collection of GasesDocumento5 páginasCollection of Gasesmalikimran2875% (4)
- Algebraic Number Theory Notes Anwar KhanDocumento110 páginasAlgebraic Number Theory Notes Anwar KhanPUBG PUBGAún no hay calificaciones
- 4 Gravitaional Constant GDocumento64 páginas4 Gravitaional Constant Ggomathi_nellaiAún no hay calificaciones
- The 55th International Mathematical Olympiad: Evan ChenDocumento33 páginasThe 55th International Mathematical Olympiad: Evan ChenYunita Septriana AnwarAún no hay calificaciones
- Cayley Contest: Canadian Mathematics CompetitionDocumento5 páginasCayley Contest: Canadian Mathematics Competitionสฮาบูดีน สาและAún no hay calificaciones
- 2019 HSSolutionDocumento16 páginas2019 HSSolutionrizky rajendra anantadewaAún no hay calificaciones
- AM GM InequalityDocumento3 páginasAM GM InequalityMorris128950Aún no hay calificaciones
- Transforming The Graphs of Trigonometric FunctionsDocumento4 páginasTransforming The Graphs of Trigonometric Functionsyddap100% (1)
- Dynamics Unit Test July 2021Documento4 páginasDynamics Unit Test July 2021Farhan HabibzaiAún no hay calificaciones
- The Chinese Remainder TheoremDocumento9 páginasThe Chinese Remainder Theoremna_hariprsadAún no hay calificaciones
- Graph Theory Soln PDFDocumento10 páginasGraph Theory Soln PDFANDRE LIM BU YUN Year14Aún no hay calificaciones
- SMO 2012 Junior QuestionDocumento8 páginasSMO 2012 Junior Questionwmdsg80% (5)
- Formulas Useful For The AMC 10 and AMC 12Documento9 páginasFormulas Useful For The AMC 10 and AMC 12johnAún no hay calificaciones
- SAT Math Level 1 Subject Test Practice Questions 1Documento32 páginasSAT Math Level 1 Subject Test Practice Questions 1Yb Andik Adi CahyonoAún no hay calificaciones
- JMC12 Web SolutionsDocumento16 páginasJMC12 Web SolutionsJuwandaAún no hay calificaciones
- 4400 Pell NotesDocumento10 páginas4400 Pell Notessticker592Aún no hay calificaciones
- 7.2 Applications of Venn DiagramsDocumento9 páginas7.2 Applications of Venn DiagramsdcevipinAún no hay calificaciones
- A Sample of A Lab ReportDocumento4 páginasA Sample of A Lab ReportDheanna Tedja100% (2)
- Chapter 1 代数综合(AMC10)Documento25 páginasChapter 1 代数综合(AMC10)Xuemei zhangAún no hay calificaciones
- Hanoi Open Mathematical Competition 2014: Senior Section Answers and SolutionsDocumento17 páginasHanoi Open Mathematical Competition 2014: Senior Section Answers and SolutionsTrưởngTrầnVănAún no hay calificaciones
- IMOmath - Polya's TheoremDocumento4 páginasIMOmath - Polya's TheoremKumar RajanAún no hay calificaciones
- Usa Aime 1984 45Documento2 páginasUsa Aime 1984 45Muhammad RizalAún no hay calificaciones
- Number Theory ProblemsDocumento9 páginasNumber Theory Problemsjoebloggs_comAún no hay calificaciones
- Number Theory Lec 3Documento2 páginasNumber Theory Lec 3Uzair KhanAún no hay calificaciones
- 60 QuestionsDocumento27 páginas60 QuestionschintusaiAún no hay calificaciones
- The Basics: Twenty-Seven ProblemsDocumento15 páginasThe Basics: Twenty-Seven ProblemskheyAún no hay calificaciones
- Let Us Get Into : Number Theory Dr. Hatem 2016Documento37 páginasLet Us Get Into : Number Theory Dr. Hatem 2016Hesham ElmasryAún no hay calificaciones
- Chapter 4 几何 Part 2(AMC10)Documento17 páginasChapter 4 几何 Part 2(AMC10)Xuemei zhangAún no hay calificaciones
- Wajo 19 QnsolnDocumento13 páginasWajo 19 QnsolnSmpnsatubontang Kaltim100% (1)
- Math HWDocumento6 páginasMath HWjarjr1Aún no hay calificaciones
- Usa Usamts 2011Documento6 páginasUsa Usamts 2011Krish KalraAún no hay calificaciones
- Factorial: Problem Code: FCTRLDocumento4 páginasFactorial: Problem Code: FCTRLNitish KumarAún no hay calificaciones
- PMWC 2018 Team Sol - EngDocumento11 páginasPMWC 2018 Team Sol - EngadiAún no hay calificaciones
- Junior Mathematical Challenge: Tuesday 30 April 2019Documento4 páginasJunior Mathematical Challenge: Tuesday 30 April 2019Add UpAún no hay calificaciones
- 2015 NZMO Olympiad SolutionsDocumento5 páginas2015 NZMO Olympiad SolutionsHoàng LongAún no hay calificaciones
- 2004 AMC 12B ProblemsDocumento5 páginas2004 AMC 12B ProblemsjabagaweeAún no hay calificaciones
- 02 03 StretchesDocumento5 páginas02 03 StretchesGladys TanAún no hay calificaciones
- 2015 Smo BookletDocumento16 páginas2015 Smo BookletMartin Martin MartinAún no hay calificaciones
- CAT - Set TheoryDocumento9 páginasCAT - Set TheoryAswath RamasubramanianAún no hay calificaciones
- Circulatory System Exhibition Lab © 2014 Vanessa Jason ("Biology Roots")Documento2 páginasCirculatory System Exhibition Lab © 2014 Vanessa Jason ("Biology Roots")p.sandoval.2027Aún no hay calificaciones
- Lab Report Ice - LFDocumento5 páginasLab Report Ice - LFRut H. P. EkasiwiAún no hay calificaciones
- Bunsen Burner LabDocumento6 páginasBunsen Burner LabGabriel Luque100% (1)
- Latent Heat of Fusion. FizikaDocumento3 páginasLatent Heat of Fusion. FizikaUgne KupryteAún no hay calificaciones
- Entropy LabDocumento6 páginasEntropy LabSiddharth RajendranAún no hay calificaciones
- Using Snell's Law To Measure The Refractive Index of PerspexDocumento5 páginasUsing Snell's Law To Measure The Refractive Index of PerspexMarc Wierzbitzki100% (4)
- Finding The Resistance of An Electrolyte (Iron Sulphate)Documento4 páginasFinding The Resistance of An Electrolyte (Iron Sulphate)Marc WierzbitzkiAún no hay calificaciones
- Investigating The Inverse Square Law For A Radioactive SourceDocumento7 páginasInvestigating The Inverse Square Law For A Radioactive SourceMarc Wierzbitzki100% (2)
- Finding The Gravitational Acceleration (G) (Atwood's Machine)Documento4 páginasFinding The Gravitational Acceleration (G) (Atwood's Machine)Marc WierzbitzkiAún no hay calificaciones
- IB Geography Oceans Case StudiesDocumento6 páginasIB Geography Oceans Case StudiesMarc Wierzbitzki0% (1)
- IB TOK Essay - "'Through Different Methods of Justification, We Can Reach Conclusions in Ethics That Are As Well-Supported As Those Provided in Mathematics.' To What Extent Would You Agree?"Documento6 páginasIB TOK Essay - "'Through Different Methods of Justification, We Can Reach Conclusions in Ethics That Are As Well-Supported As Those Provided in Mathematics.' To What Extent Would You Agree?"Marc WierzbitzkiAún no hay calificaciones
- IB Geography SL Fieldwork - An Investigation of The Effects of Longshore Drift at Two Different Beaches in Aldeburgh and ThorpenessDocumento58 páginasIB Geography SL Fieldwork - An Investigation of The Effects of Longshore Drift at Two Different Beaches in Aldeburgh and ThorpenessMarc WierzbitzkiAún no hay calificaciones
- IB Business and Management Coursework SL - Will Apple Be Able To Remain The Leader in The Tablet Market Over The Next Few Years?Documento12 páginasIB Business and Management Coursework SL - Will Apple Be Able To Remain The Leader in The Tablet Market Over The Next Few Years?Marc WierzbitzkiAún no hay calificaciones
- B747F 400Documento2 páginasB747F 400Nadeem100% (1)
- 17 Free Data Science Projects To Boost Your Knowledge & SkillsDocumento18 páginas17 Free Data Science Projects To Boost Your Knowledge & SkillshamedfazelmAún no hay calificaciones
- DP Chipset 15045 DriversDocumento592 páginasDP Chipset 15045 DriversRajesh1146Aún no hay calificaciones
- A Study On The Design Optimization of An AUV by Using Computational Fluid Dynamic AnalysisDocumento7 páginasA Study On The Design Optimization of An AUV by Using Computational Fluid Dynamic AnalysisSalma SherbazAún no hay calificaciones
- ARSTRUCTS Chapter1Documento15 páginasARSTRUCTS Chapter1Aila MaeAún no hay calificaciones
- Steam Boiler Technology (2003)Documento218 páginasSteam Boiler Technology (2003)Majid Sattar100% (3)
- Grid Synchronization of Power Converters Using Multiple Second Order Generalized IntegratorsDocumento6 páginasGrid Synchronization of Power Converters Using Multiple Second Order Generalized IntegratorsJandfor Tansfg ErrottAún no hay calificaciones
- Lectures - Mass TransferDocumento36 páginasLectures - Mass TransferaaaAún no hay calificaciones
- Lab Gas FlowmeterDocumento7 páginasLab Gas Flowmeterazym94Aún no hay calificaciones
- Opus UserguideDocumento313 páginasOpus UserguideMoez EssafiAún no hay calificaciones
- 9.16. Prepare A Plot of Work Per Pound Mole Versus The Pressue Ratio PDocumento6 páginas9.16. Prepare A Plot of Work Per Pound Mole Versus The Pressue Ratio PttussenoAún no hay calificaciones
- LG Wd1873rds Manual de UsuarioDocumento76 páginasLG Wd1873rds Manual de UsuarioJosè Otoniel Osorio BarreraAún no hay calificaciones
- Graco Pumps Catalog 300435EN MDocumento76 páginasGraco Pumps Catalog 300435EN MAlbu MihaiAún no hay calificaciones
- Catalogo Towel RailsDocumento1 páginaCatalogo Towel RailsrodijammoulAún no hay calificaciones
- Subsea AccumulatorsDocumento4 páginasSubsea AccumulatorsAbdul Hameed OmarAún no hay calificaciones
- TK-C Transmitter PDFDocumento4 páginasTK-C Transmitter PDFGopal HegdeAún no hay calificaciones
- 16PPE723Documento2 páginas16PPE723DrArun KaliappanAún no hay calificaciones
- POH NAVAJO Pa-31Documento438 páginasPOH NAVAJO Pa-31Mantenimiento CMA68475% (4)
- Debug 1214Documento3 páginasDebug 1214Anonymous B4WiRjAún no hay calificaciones
- Digital Pressure Gauge XP2i PSI Data Sheet USDocumento5 páginasDigital Pressure Gauge XP2i PSI Data Sheet USAbdurrachman JalaludinAún no hay calificaciones
- Cfw300 Manual 1Documento124 páginasCfw300 Manual 1maurilioAún no hay calificaciones
- Uptake and Distribution of Inhalational AnaestheticsDocumento125 páginasUptake and Distribution of Inhalational Anaestheticsharsha mummakaAún no hay calificaciones
- KYK CatalogueDocumento94 páginasKYK Cataloguepriya kumariAún no hay calificaciones
- Arup Scheme Design GuideDocumento139 páginasArup Scheme Design GuideDean TyrrellAún no hay calificaciones
- 10SQ050 PDFDocumento3 páginas10SQ050 PDFprojects eastlinkAún no hay calificaciones
- Surface Condenser Eng2Documento5 páginasSurface Condenser Eng2MuhammadFikriAún no hay calificaciones
- PDFDocumento255 páginasPDFwrite2arshad_mAún no hay calificaciones
- 02 - Student Lesson 2 Pile Driving SystemDocumento91 páginas02 - Student Lesson 2 Pile Driving SystemdannyzuanAún no hay calificaciones
- Flight DynamicsDocumento57 páginasFlight DynamicsDexto100% (2)
- Sagar Ovhalkar (Site)Documento2 páginasSagar Ovhalkar (Site)Dayanand WasateAún no hay calificaciones
- Giza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyDe EverandGiza: The Tesla Connection: Acoustical Science and the Harvesting of Clean EnergyAún no hay calificaciones
- A Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceDe EverandA Beginner's Guide to Constructing the Universe: The Mathematical Archetypes of Nature, Art, and ScienceCalificación: 4 de 5 estrellas4/5 (51)
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseDe EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseCalificación: 3.5 de 5 estrellas3.5/5 (69)
- Knocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldDe EverandKnocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldCalificación: 3.5 de 5 estrellas3.5/5 (64)
- The Beauty of Falling: A Life in Pursuit of GravityDe EverandThe Beauty of Falling: A Life in Pursuit of GravityAún no hay calificaciones
- Summary and Interpretation of Reality TransurfingDe EverandSummary and Interpretation of Reality TransurfingCalificación: 5 de 5 estrellas5/5 (5)
- The End of Everything: (Astrophysically Speaking)De EverandThe End of Everything: (Astrophysically Speaking)Calificación: 4.5 de 5 estrellas4.5/5 (157)
- Midnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterDe EverandMidnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterCalificación: 4.5 de 5 estrellas4.5/5 (410)
- A Brief History of Time: From the Big Bang to Black HolesDe EverandA Brief History of Time: From the Big Bang to Black HolesCalificación: 4 de 5 estrellas4/5 (2193)
- Higgs Discovery: The Power of Empty SpaceDe EverandHiggs Discovery: The Power of Empty SpaceCalificación: 3 de 5 estrellas3/5 (30)
- The Holographic Universe: The Revolutionary Theory of RealityDe EverandThe Holographic Universe: The Revolutionary Theory of RealityCalificación: 4.5 de 5 estrellas4.5/5 (78)
- The Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeDe EverandThe Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeAún no hay calificaciones
- Lost in Math: How Beauty Leads Physics AstrayDe EverandLost in Math: How Beauty Leads Physics AstrayCalificación: 4.5 de 5 estrellas4.5/5 (125)
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessDe EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessCalificación: 4 de 5 estrellas4/5 (6)
- Bedeviled: A Shadow History of Demons in ScienceDe EverandBedeviled: A Shadow History of Demons in ScienceCalificación: 5 de 5 estrellas5/5 (5)
- The Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldDe EverandThe Power of Eight: Harnessing the Miraculous Energies of a Small Group to Heal Others, Your Life, and the WorldCalificación: 4.5 de 5 estrellas4.5/5 (54)
- The Beginning of Infinity: Explanations That Transform the WorldDe EverandThe Beginning of Infinity: Explanations That Transform the WorldCalificación: 5 de 5 estrellas5/5 (60)
- Quantum Physics: What Everyone Needs to KnowDe EverandQuantum Physics: What Everyone Needs to KnowCalificación: 4.5 de 5 estrellas4.5/5 (49)
- Quantum Physcis for Beginners: An Easy Guide for Discovering the Hidden Side of Reality One Speck at a TimeDe EverandQuantum Physcis for Beginners: An Easy Guide for Discovering the Hidden Side of Reality One Speck at a TimeAún no hay calificaciones
- Vibration and Frequency: How to Get What You Want in LifeDe EverandVibration and Frequency: How to Get What You Want in LifeCalificación: 4.5 de 5 estrellas4.5/5 (13)
- Hyperspace: A Scientific Odyssey Through Parallel Universes, Time Warps, and the 10th DimensionDe EverandHyperspace: A Scientific Odyssey Through Parallel Universes, Time Warps, and the 10th DimensionCalificación: 4.5 de 5 estrellas4.5/5 (3)
- Packing for Mars: The Curious Science of Life in the VoidDe EverandPacking for Mars: The Curious Science of Life in the VoidCalificación: 4 de 5 estrellas4/5 (1396)
- The Simulated Multiverse: An MIT Computer Scientist Explores Parallel Universes, The Simulation Hypothesis, Quantum Computing and the Mandela EffectDe EverandThe Simulated Multiverse: An MIT Computer Scientist Explores Parallel Universes, The Simulation Hypothesis, Quantum Computing and the Mandela EffectCalificación: 4.5 de 5 estrellas4.5/5 (20)
- Transform Your Life And Save The World: Through The Dreamed Of Arrival Of The Rehabilitating Biological Explanation Of The Human ConditionDe EverandTransform Your Life And Save The World: Through The Dreamed Of Arrival Of The Rehabilitating Biological Explanation Of The Human ConditionCalificación: 5 de 5 estrellas5/5 (2)
- Let There Be Light: Physics, Philosophy & the Dimensional Structure of ConsciousnessDe EverandLet There Be Light: Physics, Philosophy & the Dimensional Structure of ConsciousnessCalificación: 4.5 de 5 estrellas4.5/5 (57)