Documentos de Académico
Documentos de Profesional
Documentos de Cultura
Determination of Vitamin C
Cargado por
Walwin HareDerechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Determination of Vitamin C
Cargado por
Walwin HareCopyright:
Formatos disponibles
DETERMINATION OF VITAMIN C IN a REDOXON TABLET
This application exploits the absorbing tendencies of certain compounds towards light in
order to determine their concentration in solution. Certain species absorbs light at a
specified wavelength from which the concentration of the absorbing species can be found
out. According to the Beer-Lambert law (A=Ecl); where A is the absorbance, E refers to
the extinction coefficient, c the concentration and l the path length, the unknown
concentration of an absorbing species can be found by plotting a graph of absorbance vs.
concentration. A simple rearrangement of the formula gives the concentration. This is
applied in the case of wanting to find out the concentration of vitamin c in a Redoxon
tablet.
METHODOLOGY
REAGENTS TO BE USED
Prepare a 2000ugmL-1 solution of ascorbic acid by dissolving 2.0g of ascorbic acid in a
1000ml volumetric flask and making it up to the mark. Also prepare a 200ugmL-1 of a
CuSO4 solution by dissolving 0.2g of CuSO4 in 0.02 moldm-3 H2SO4. Prepare also a
60ugmL-1 of copper solution, 0.2% w/v 2, 9-dimethyl-1, 10-phenanthroline (neucoproine)
solution prepared in 90% v/v ethyl alcohol, a pH of 7.5 buffer solutions to maintain the
pH, 0.4% w/v N-phenylbenzimidoylthiourea (PBITU) in chloroform for the extraction of
copper.2
PROCEDURE
Weigh out 1.0 g of Redoxon tablet on an analytical balance in a 5ml beaker. Transfer the
tablet to a 50ml beaker and dissolve it in about 40cm3 of water. Stir the solution with a
glass rod until the entire tablet has dissolved; this will be indicated by a colourless
solution. After this, filter through a Whatmann filter paper. A 1mL sample should then be
added and analyzed by following the steps hereafter. An aliquot of standard solution
containing 1.0-5.0ugmL-1 of ascorbic acid is poured into a 125ml separatory funnel. To
this solution, 3mL of copper solution, 3ML buffer solution and 2mL neucoproine solution
is added. Following this is the dilution of the aqueous phase to 20mL with distilled
water.2 This will result in the formation of a complex which is then extracted with 20mL
chloroform solution of PBITU for 3mins. The aqueous phase should then be run-off and
the colour of the extract measured at a maximum wavelength of 480nm using a blank
solution as the reference, after it has been dried over anhydrous sodium sulphate (~3gm).
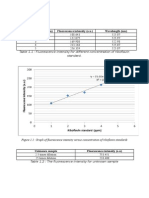
The concentration of the ascorbic acid can then be determined by using the standard
calibration curve method.2
MANUFACTURER
A suitable manufacturer for this instrument is Systronics. The instrument is the uv-vis
spectrophotometer model 108. It has a bandwidth of 0.9nm and is PMT based.1
REFERENCE
1) http://www.systronicsindia.com/templates/systronics/images/pro/UV
%20Visible%20Spectrophotometer/1.pdf
2) Journal of the Chinese Chemical Society, 2005, 52, 503-506
También podría gustarte
- Introductory Titrimetric and Gravimetric Analysis: The Commonwealth and International Library: Chemistry DivisionDe EverandIntroductory Titrimetric and Gravimetric Analysis: The Commonwealth and International Library: Chemistry DivisionAún no hay calificaciones
- Complex SaltDocumento8 páginasComplex Saltmipa amarAún no hay calificaciones
- Measurements of Surface TensionDocumento11 páginasMeasurements of Surface TensionHema ParasuramanAún no hay calificaciones
- NaBH4 Reduction of Cyclohexanone to Cyclohexanol (87Documento8 páginasNaBH4 Reduction of Cyclohexanone to Cyclohexanol (87hahadindongAún no hay calificaciones
- Kinetics 1Documento3 páginasKinetics 1JuarezAún no hay calificaciones
- Experi Men 22Documento7 páginasExperi Men 22bernardAún no hay calificaciones
- Finals PhychemDocumento3 páginasFinals PhychemniezajanepatnaAún no hay calificaciones
- Fluoride Ion Selective ElectrodeDocumento14 páginasFluoride Ion Selective ElectrodeMihEugen100% (1)
- Inorganic Prac 2Documento3 páginasInorganic Prac 2Ray DyerAún no hay calificaciones
- Measure Water ConductivityDocumento7 páginasMeasure Water ConductivitySilvy SaavedraAún no hay calificaciones
- EXPERIMENT 2 Reduction of CamphorDocumento2 páginasEXPERIMENT 2 Reduction of CamphorDania FaridAún no hay calificaciones
- Lab Report - IsE FluorinityDocumento5 páginasLab Report - IsE FluorinityJohn LamAún no hay calificaciones
- Electrogravimetry: A Three-Electrode ApproachDocumento11 páginasElectrogravimetry: A Three-Electrode ApproachBang100% (1)
- Exp 6Documento6 páginasExp 6MsShu93100% (1)
- The Fluoride Ion Selective Electrode ExperimentDocumento5 páginasThe Fluoride Ion Selective Electrode Experimentlisaaliyo0% (1)
- 4A F16 Exp 04 Absorbance and Fluorescence Spectros PDFDocumento27 páginas4A F16 Exp 04 Absorbance and Fluorescence Spectros PDFWanqing HeAún no hay calificaciones
- Lab Report (Final Editied)Documento8 páginasLab Report (Final Editied)Alexia Channer100% (4)
- PH CH 126.1 Fischer Esterification of Methyl Benzoate 2Documento6 páginasPH CH 126.1 Fischer Esterification of Methyl Benzoate 2Tammy CacnioAún no hay calificaciones
- Experiment 3 Redox Titration Percent Purity AnalysisDocumento5 páginasExperiment 3 Redox Titration Percent Purity AnalysisnanaAún no hay calificaciones
- OPTICAL ACTIVITY OF SUCROSE AND CYSTEINEDocumento4 páginasOPTICAL ACTIVITY OF SUCROSE AND CYSTEINEJohn Mark Flores Villena100% (1)
- Heat of SolutionDocumento1 páginaHeat of Solutionsimonatics08Aún no hay calificaciones
- Experiment 2 Sodium Borohydride Reduction of CyclohexanoneDocumento6 páginasExperiment 2 Sodium Borohydride Reduction of CyclohexanoneSarah HannisAún no hay calificaciones
- Exp 2 Redox Inorganic ChemistryDocumento11 páginasExp 2 Redox Inorganic ChemistryAhmad Rawi100% (1)
- Practice Problems For Physical Chemistry 2Documento1 páginaPractice Problems For Physical Chemistry 2Fatima CellonaAún no hay calificaciones
- Preparation of Potassium TrioxaloferrateDocumento10 páginasPreparation of Potassium Trioxaloferratemukund_seethamrajuAún no hay calificaciones
- Uv Vis Spectroscopy Lab Report of The Detection of Analytes From Environmental Samples Using UvDocumento13 páginasUv Vis Spectroscopy Lab Report of The Detection of Analytes From Environmental Samples Using UvSaba Naseer100% (1)
- Determination of Copper by AASDocumento18 páginasDetermination of Copper by AASWan ShamAún no hay calificaciones
- Synthesis of TetraaminecopperDocumento4 páginasSynthesis of Tetraaminecopperrahma0% (1)
- 14 - Lab 14 - R-HPLC For Detn of CaffeineDocumento7 páginas14 - Lab 14 - R-HPLC For Detn of CaffeineHoang Huong TraAún no hay calificaciones
- Electrochemistry: Introduction To Galvanic Cells and Nernst EquationDocumento3 páginasElectrochemistry: Introduction To Galvanic Cells and Nernst EquationTinuviele EsguerraAún no hay calificaciones
- Field EmissionDocumento6 páginasField Emissionle_fridaAún no hay calificaciones
- Determination of Fluoride Concentration Using Ion Selective ElectrodeDocumento7 páginasDetermination of Fluoride Concentration Using Ion Selective ElectrodeAmanda WangAún no hay calificaciones
- Riboflavin Concentration Fluorescence SpectroscopyDocumento5 páginasRiboflavin Concentration Fluorescence SpectroscopySyahriezan HaminAún no hay calificaciones
- Vpma 3 (4C - Clerkship Program) : (1 Point)Documento21 páginasVpma 3 (4C - Clerkship Program) : (1 Point)Prabin ChaudharyAún no hay calificaciones
- Introduction to analytical Separations techniquesDocumento43 páginasIntroduction to analytical Separations techniquesVel MuruganAún no hay calificaciones
- Exp 6Documento8 páginasExp 6KaVisha AShaAún no hay calificaciones
- Complex SaltDocumento29 páginasComplex SaltertaAún no hay calificaciones
- PolypyrroleDocumento106 páginasPolypyrrolesurya rajAún no hay calificaciones
- Ferric OxalateDocumento11 páginasFerric OxalateJoao Diniz100% (1)
- IA Report Determination of Metals With ICP Atomic Emission SpectrometryDocumento8 páginasIA Report Determination of Metals With ICP Atomic Emission SpectrometrySaranya KannanAún no hay calificaciones
- Job's Method Determination of Complex StoichiometryDocumento3 páginasJob's Method Determination of Complex StoichiometryVaid RahulAún no hay calificaciones
- Aspirin Uv VisDocumento6 páginasAspirin Uv VisseanAún no hay calificaciones
- Kinetics of Iodination of Acetone Catalyzed by HCL and H2so4 A Colorimetric Investigation of Relative Strength PDFDocumento4 páginasKinetics of Iodination of Acetone Catalyzed by HCL and H2so4 A Colorimetric Investigation of Relative Strength PDFHansel VereitelnAún no hay calificaciones
- Ioron Determination in WaterDocumento6 páginasIoron Determination in WaterGobe JamAún no hay calificaciones
- Spectrophotometric Determination of Phosphate in Detergent: Santos, Nanette, D., Ortega, Mary Alyssa, TDocumento4 páginasSpectrophotometric Determination of Phosphate in Detergent: Santos, Nanette, D., Ortega, Mary Alyssa, TAlyssa OrtegaAún no hay calificaciones
- Determination of Na and K by flame photometryDocumento2 páginasDetermination of Na and K by flame photometryDebleena ChakrabortyAún no hay calificaciones
- Measuring Manganese Using SpectrophotometryDocumento8 páginasMeasuring Manganese Using SpectrophotometryCuprum29Aún no hay calificaciones
- Vibration - Rotation Spectroscopy of HCL and DCLDocumento9 páginasVibration - Rotation Spectroscopy of HCL and DCLAngela LamasAún no hay calificaciones
- Electrogravimetric Analysis of CopperDocumento2 páginasElectrogravimetric Analysis of Coppervandv printsAún no hay calificaciones
- Carbohydrates: Color Reactions and TestsDocumento19 páginasCarbohydrates: Color Reactions and TestsAjith KumarAún no hay calificaciones
- Full Report Carbs On 161.1Documento23 páginasFull Report Carbs On 161.1Kim Leonard BolandosAún no hay calificaciones
- Experiment 2 Uv-Visible Determination of An Unknown Concentration of Kmno Solution Theory/BackgroundDocumento13 páginasExperiment 2 Uv-Visible Determination of An Unknown Concentration of Kmno Solution Theory/BackgroundMuhammad Azri HaziqAún no hay calificaciones
- Lab Manual Yr 3 InorganicDocumento33 páginasLab Manual Yr 3 InorganicOmSilence2651Aún no hay calificaciones
- Transition Metal ToxicityDe EverandTransition Metal ToxicityG. W. RichterAún no hay calificaciones
- Essays on Analytical Chemistry: In Memory of Professor Anders RingbomDe EverandEssays on Analytical Chemistry: In Memory of Professor Anders RingbomErkki WänninenAún no hay calificaciones
- Experiments in Physical Chemistry: Second Revised and Enlarged EditionDe EverandExperiments in Physical Chemistry: Second Revised and Enlarged EditionAún no hay calificaciones
- Ion Exchange in Analytical Chemistry: International Series of Monographs in Analytical ChemistryDe EverandIon Exchange in Analytical Chemistry: International Series of Monographs in Analytical ChemistryCalificación: 5 de 5 estrellas5/5 (1)
- Columns for Gas Chromatography: Performance and SelectionDe EverandColumns for Gas Chromatography: Performance and SelectionAún no hay calificaciones
- Effect of Water Potential On Plant CellDocumento2 páginasEffect of Water Potential On Plant CellWalwin HareAún no hay calificaciones
- Acetic Acid Content of Vinegar P&D LabDocumento2 páginasAcetic Acid Content of Vinegar P&D LabWalwin Hare56% (16)
- Effect of Water Potential On Plant CellDocumento2 páginasEffect of Water Potential On Plant CellWalwin HareAún no hay calificaciones
- Osmosis Discussion LabDocumento1 páginaOsmosis Discussion LabWalwin Hare50% (4)
- Effect of Heat On Vitamin C Content P&D LabDocumento1 páginaEffect of Heat On Vitamin C Content P&D LabWalwin Hare50% (2)
- Enzyme Lab - Effect of Concentration On RateDocumento1 páginaEnzyme Lab - Effect of Concentration On RateWalwin HareAún no hay calificaciones
- Food Test For Unknown Food SamplesDocumento2 páginasFood Test For Unknown Food SamplesWalwin HareAún no hay calificaciones
- Energy Content of FoodsDocumento2 páginasEnergy Content of FoodsWalwin Hare75% (4)
- Digestion ConciseDocumento3 páginasDigestion ConciseWalwin HareAún no hay calificaciones
- Bio ManualDocumento4 páginasBio ManualWalwin HareAún no hay calificaciones
- Soil LabDocumento1 páginaSoil LabWalwin HareAún no hay calificaciones
- Enzyme Lab - Effect of PHDocumento2 páginasEnzyme Lab - Effect of PHWalwin HareAún no hay calificaciones
- DNA Fingerprinting & Forensic Science MethodsDocumento4 páginasDNA Fingerprinting & Forensic Science MethodsWalwin HareAún no hay calificaciones
- Photosynthesis Rate & Light IntensityDocumento2 páginasPhotosynthesis Rate & Light IntensityWalwin HareAún no hay calificaciones
- Definition of The Caribbean EssayDocumento2 páginasDefinition of The Caribbean EssayWalwin Hare82% (28)
- Molecular Biology ExperimentDocumento6 páginasMolecular Biology ExperimentWalwin Hare100% (1)
- Enzyme Lab - Effect of Temperature On RateDocumento1 páginaEnzyme Lab - Effect of Temperature On RateWalwin HareAún no hay calificaciones
- High Pressure Liquid ChromatographyDocumento5 páginasHigh Pressure Liquid ChromatographyWalwin HareAún no hay calificaciones
- Analysis of pTrc99A/topA DNA Using Restriction EnzymesDocumento3 páginasAnalysis of pTrc99A/topA DNA Using Restriction EnzymesWalwin Hare60% (5)
- HFCS & ObesityDocumento16 páginasHFCS & ObesityWalwin HareAún no hay calificaciones
- The Class ReptiliaDocumento2 páginasThe Class ReptiliaWalwin Hare0% (1)
- Essay On The Characteristics of AmphibiansDocumento2 páginasEssay On The Characteristics of AmphibiansWalwin HareAún no hay calificaciones
- How Birds Are Adapted For FlightDocumento3 páginasHow Birds Are Adapted For FlightWalwin HareAún no hay calificaciones
- Teleosts (Fish)Documento4 páginasTeleosts (Fish)Walwin HareAún no hay calificaciones
- Martines Krista Sum2015Documento26 páginasMartines Krista Sum2015Ariel Carl Angelo BalletaAún no hay calificaciones
- Energy Series Dynamic Dome SkylightsDocumento2 páginasEnergy Series Dynamic Dome SkylightsoutmatchAún no hay calificaciones
- Incidence of Candidiasis Amongst Female Students of A Tertiary Institution in Rivers State NigeriaDocumento6 páginasIncidence of Candidiasis Amongst Female Students of A Tertiary Institution in Rivers State NigeriaUbali Ibrahim HashimuAún no hay calificaciones
- Fair movement in Ukraine has centuries-long historyDocumento6 páginasFair movement in Ukraine has centuries-long historyLidia LytvynenkoAún no hay calificaciones
- Buoyancy: Archimedes' PrincipleDocumento2 páginasBuoyancy: Archimedes' PrincipleKevin SmallAún no hay calificaciones
- GR 126010 Hernandez Vs Hernandez SHLD Be Legal SepDocumento1 páginaGR 126010 Hernandez Vs Hernandez SHLD Be Legal SepMichael JonesAún no hay calificaciones
- Kel 2 - Hospital DischargeDocumento18 páginasKel 2 - Hospital DischargeFeby KartikasariAún no hay calificaciones
- G8 Long QuizDocumento3 páginasG8 Long QuizJubylyn AficialAún no hay calificaciones
- Chapter 7 - Muscuar SystemDocumento17 páginasChapter 7 - Muscuar SystemM GarciaAún no hay calificaciones
- Year 9 - Justrice System Civil LawDocumento12 páginasYear 9 - Justrice System Civil Lawapi-301001591Aún no hay calificaciones
- Relationship between cytokines and hazards in waste workersDocumento8 páginasRelationship between cytokines and hazards in waste workersSalsa BilaAún no hay calificaciones
- T S Form 3Documento2 páginasT S Form 3Lubinda Lubinda Jr.Aún no hay calificaciones
- UT Dallas Syllabus For Biol3350.501 06s Taught by Ilya Sapozhnikov (Isapoz)Documento4 páginasUT Dallas Syllabus For Biol3350.501 06s Taught by Ilya Sapozhnikov (Isapoz)UT Dallas Provost's Technology GroupAún no hay calificaciones
- (PPT) Arresting Cracks in Steel BridgesDocumento61 páginas(PPT) Arresting Cracks in Steel BridgesShaileshRastogiAún no hay calificaciones
- Preprint Not Peer Reviewed: A Review of Domestic Violence Against Women in India During LockdownDocumento13 páginasPreprint Not Peer Reviewed: A Review of Domestic Violence Against Women in India During LockdownAishwarya MoitraAún no hay calificaciones
- Pelvic AnatomyDocumento106 páginasPelvic AnatomyRosu George100% (1)
- Daftar Nama DistributorDocumento12 páginasDaftar Nama DistributorAny Moneta SariAún no hay calificaciones
- Effect of pumpkin flour on characteristics of chiffon cake made from modified cassava flourDocumento12 páginasEffect of pumpkin flour on characteristics of chiffon cake made from modified cassava flourNurul HasanahAún no hay calificaciones
- Fabrication & Installation of Thorp T-211 WingDocumento102 páginasFabrication & Installation of Thorp T-211 WingSudharSanAún no hay calificaciones
- OUR Company: MGT 351 Presentation On Human Resource PlanDocumento16 páginasOUR Company: MGT 351 Presentation On Human Resource Planaparajita promaAún no hay calificaciones
- Manfaat Yang Didapatkan Mahasiswa Dalam Mengikuti: Interprofessional Education (Ipe) Dengan Pendekatan Case StudyDocumento5 páginasManfaat Yang Didapatkan Mahasiswa Dalam Mengikuti: Interprofessional Education (Ipe) Dengan Pendekatan Case StudyganditAún no hay calificaciones
- Chapter 2 LaboratoryDocumento6 páginasChapter 2 LaboratoryVea Roanne GammadAún no hay calificaciones
- Spotters 1Documento10 páginasSpotters 1elavarkuzhali2019Aún no hay calificaciones
- A Sonographic Sign of Moderate ToDocumento5 páginasA Sonographic Sign of Moderate ToDivisi FER MalangAún no hay calificaciones
- Spa Multiple PrincipalDocumento2 páginasSpa Multiple PrincipalGn67% (3)
- UD-800 BR WDocumento2 páginasUD-800 BR WFrancisco GomezAún no hay calificaciones
- BandhaDocumento3 páginasBandhaMouli ChandraAún no hay calificaciones
- First Aid Basics and Vital Signs EssentialsDocumento18 páginasFirst Aid Basics and Vital Signs EssentialsJoyce Anne Tuala YabutAún no hay calificaciones
- Ground Improvement Techniques PDFDocumento54 páginasGround Improvement Techniques PDFMohit Rajai67% (6)
- NGU Fans Technical SubmittalsDocumento104 páginasNGU Fans Technical SubmittalsAhmed NabilAún no hay calificaciones