Documentos de Académico
Documentos de Profesional
Documentos de Cultura
Lupus Nephritis
Cargado por
sobanDerechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Lupus Nephritis
Cargado por
sobanCopyright:
Formatos disponibles
SYSTEMIC DISEASE AND THE KIDNEY
Whats new ?
Lupus Nephritis
Despite immunosuppressive therapy, lupus nephritis
remains a strong predictor of ESRF and death in SLE
Jane Cross
David Jayne
Mycophenolate mofetil is a newer immunosuppressive
with an emerging role in lupus nephritis
Systemic lupus erythematosus (SLE) is a multisystem autoimmune
disease of unknown cause (see MEDICINE 30:10, 6). In the UK,
the prevalence is 27/100,000; the disease predominantly occurs
in women under the age of 40 years, though any age group may
be affected. Overt renal disease occurs in at least one-third of SLE
patients and is the most common severe manifestation. It is one of
the eleven diagnostic criteria proposed by the American College of
Rheumatology, four of which are required to support a diagnosis
of SLE. Development of nephritis is closely linked to survival and
morbidity; 20% of patients die and 25% reach end-stage renal
failure (ESRF) within 10 years. However, there is considerable
variation in presentation, pathology, course and outcome. Lupus
nephritis responds to corticosteroid and immunosuppressive
therapy, but the toxicity of current drugs contributes to morbidity
and mortality.
glomerular sclerosis. Extraglomerular features of lupus nephritis
include tubulo-interstitial nephritis (70% of patients), renal vein
thrombosis and renal artery stenosis. Thrombotic manifestations
are associated with autoantibodies to phospholipids, which are
detectable as circulating anticardiolipin autoantibodies or the
lupus anticoagulant.
Clinical features and investigations
Only 25% of SLE patients present with renal disease as the first
manifestation of the condition. In 5% of cases (usually men
> 40 years), it is several years before other criteria or serological abnormalities develop. Patients may present with asymptomatic urinary abnormalities such as microscopic haematuria or
proteinuria detected on routine testing in established SLE, with
hypertension, or with nephrotic syndrome (40%). Less commonly,
lupus nephritis presents as acute renal failure with symptoms of
renal impairment, in which case other severe manifestations (e.g.
myocarditis, cerebritis) may also be present.
The following factors influence the course of lupus nephritis
and its outcome, and should be considered in the evaluation of
patients:
demography (age, sex, race, duration of SLE and nephritis)
renal function (glomerular filtration rate, urinary abnormalities,
blood pressure)
serology (autoantibodies, complement, immunoglobulins,
albumin)
histopathology (light microscopy, immunofluorescence)
extrarenal organ involvement and drug exposure.
The histological appearances of glomerular disease have been
classified according to the pattern and extent of immune deposition and inflammation (Figure 1). Transformation to a more
severe or less severe histological class is well documented and
may result from treatment or be part of the natural history of the
disease. The activity and chronicity of renal biopsy specimens
are used to assess whether treatment should be intensified, and
chronicity indices may predict the long-term renal outcome. Interpretation of renal biopsy is subject to observer bias, however, and
is influenced by the sample size.
Pathology
Immune deposits in the glomeruli and mesangium are characteristic of SLE, and stain positive for IgG, IgM, IgA and the complement components C3, C1q and C4 on immunofluorescence. Circulating autoantibodies to cellular antigens (particularly anti-dsDNA,
anti-Ro and anti-C1q) and complement activation with correspondingly reduced serum C3, C4 and C1q levels are typical of lupus
nephritis. Following the appearance of immune complexes, an
inflammatory reaction develops with mesangial cell proliferation,
expansion of the mesangial matrix and infiltration of inflammatory
leucocytes. Other pathogenic mechanisms include infarction of
glomerular segments, thrombotic microangiopathy, vasculitis and
Jane Cross is a Renal Registrar at Addenbrookes Hospital, Cambridge,
UK. She qualified from the Royal London Hospital, London, and has
trained in the South West Thames region. Her interests include systemic
lupus erythematosus and trial design.
Management
David Jayne is Consultant Physician and Director of the Vasculitis and
Lupus Clinic at Addenbrookes Hospital, Cambridge, UK. He qualified
from the University of Cambridge and St Thomas Hospital, London,
and trained in general medicine and nephrology. His research interests
include immunotherapy and clinical trials in vasculitis and systemic
lupus erythematosus.
MEDICINE
No large, prospective randomized trials have been performed in
lupus nephritis; data are derived from retrospective studies and
from smaller trials with an average of 20 patients per treatment
arm. Most data suggest that WHO class II lupus nephritis has a
benign course, and treatment in the absence of other indications
101
2003 The Medicine Publishing Company Ltd
SYSTEMIC DISEASE AND THE KIDNEY
Survival to end-stage renal failure or death in lupus
nephritis according to histological grade
Modified WHO classification of lupus nephritis
Class 0
Normal
Class II
1.0
Class I
Light microscopy normal, immune deposits on immunofluorescence
Class V
0.8
Class II
A mesangial deposits
B mesangial hypercellularity
Class III
Class I
Survival
0.6
Class III
Focal segmental proliferative glomerulonephritis (< 50% glomeruli)
with leucocyte infiltration; immune deposits in the mesangium and
subendothelium
Class IV
0.4
Class IV
As class III, but diffuse (> 50% glomeruli); may also see glomerular
crescents and fibrinoid necrosis; includes membranoproliferative
variant
0.2
Class VI
Class V
Membranous nephropathy with uniform thickening of the capillary
walls and subepithelial immune complex deposition
A membranous nephropathy alone
B membranous nephropathy + class II
C membranous nephropathy + class III
D membranous nephropathy + class IV
10
15
20
Time after biopsy (years)
Histological grade predicts subsequent development of renal failure in
lupus nephritis. The current grading system does not take into account the
full range of lupus manifestations in the kidney, but remains the most
widely used tool. Glomerulofibrosis is the most important predictor of renal
failure, which is reflected in the poor outcome in WHO class VI nephritis.
(By courtesy of Dr A Howie and Dr D Adu, Queen Elizabeth II Hospital,
Birmingham.)
Class VI
Glomerulosclerosis
2
1
suppression and infection are lower than with cyclophosphamide,
but there is an increased risk of skin malignancy after prolonged
exposure.
Treatment-related death and morbidity from infection are significant problems in SLE, and newer, less toxic agents are sought.
Mycophenolate mofetil is used for both induction of remission and
maintenance therapy. Autologous peripheral stem cell transplantation is under evaluation for refractory and relapsing disease.
is usually not required. The outcome and treatment of class V
disease is debated, reflecting differences in interpretation of the
histological criteria.
The decision to treat active WHO class III and IV lupus nephritis
is less controversial. Systematic review of the available trials supports treatment with corticosteroids and an immunosuppressive
agent. The choice of immunosuppression is less clear; traditionally,
cyclophosphamide or azathioprine is used, but neither has been
shown to be superior to the other. In the UK, pulsed intravenous
cyclophosphamide with corticosteroids is the most widely used
first-line therapy to induce remission of active nephritis. Early
withdrawal of immunosuppression increases the relapse rate, so
cyclophosphamide is either continued or substituted by the less
toxic azathioprine. The optimum duration of therapy is debated;
continuing treatment for a significant disease-free period such as
2 years is recommended. Ciclosporin is an alternative immunosuppressive agent, used particularly in children.
Both cyclophosphamide and azathioprine have severe adverse
effects. Cyclophosphamide is associated with premature menopause in up to 50% of women, myelosuppression, an increased
risk of severe infections and bladder malignancy. Azathioprine
is associated with hypersensitivity reactions. The risks of myelo-
MEDICINE
Prognosis
Development of renal disease is the strongest predictor of ESRF and
early mortality in SLE. Renal histology and renal function are the
most important renal prognostic factors at presentation (Figure 2).
Following treatment, normalization of proteinuria and the absence
of relapse of nephritis are the best predictors of a good outcome.
Negative prognostic factors include male gender, black race and
haematological features of SLE.
Pregnancy and lupus nephritis
The effect of pregnancy on the activity of SLE is debated, but
disease activity at conception is important fetal loss is greater in
women with active disease at this time. Renal impairment per se
102
2003 The Medicine Publishing Company Ltd
SYSTEMIC DISEASE AND THE KIDNEY
is also associated with fetal loss and premature delivery. Management by a specialist team before conception and during pregnancy
is important in optimizing fetal and renal outcome.
u
Drugs and
Renal Insufficiency
REFERENCES
Balow J E, Boumpas D T, Fessler B J et al. Management of Lupus Nephritis.
Kidney Int 1996; 49: (Suppl. 53): 8892.
Bansal V K, Beto J A. Treatment of Lupus Nephritis: A Meta-analysis of
Clinical Trials. Am J Kidney Dis 1997; 29(2): 1939.
Hochberg M C. Updating the American College of Rheumatology Revised
Criteria for the Classification of Systemic Lupus Erythematosus.
Arthritis Rheum 1997; 40(9): 1725.
Korbet S M, Lewis E J, Schwartz M M et al. Factors Predictive of
Outcome in Severe Lupus Nephritis. Am J Kidney Dis 2000; 35(5):
90414.
Jeffrey K Aronson
Renal insufficiency alters both the disposition of drugs in the body
(pharmacokinetics) and tissue responses to drugs (pharmacodynamics), and nephrotoxic drugs can impair renal function.
Pharmacokinetic changes include the following.
Drugs that are excreted by the kidneys in an active form or
as active metabolites are eliminated at a reduced rate. Reduced
clearance causes accumulation of the drug, which can cause adverse effects. Renal replacement therapy can also alter the rate of
elimination of drugs.
Altered protein binding occurs. Displacement (e.g. phenytoin)
increases the unbound fraction of drug available for distribution
to the tissues and enhances its action.
Diuretics that act on the luminal side of the renal tubule do
not reach their site of action in sufficiently high concentrations;
increased doses are therefore required.
FURTHER READING
Davison A M, Cameron J S, Grnfeld J-P et al., eds. Oxford Textbook of
Clinical Nephrology. Vol. 2. 2nd ed. Oxford: Oxford University Press,
1998.
(Includes an excellent review of all aspects of lupus nephritis.)
Balow J E, Austin H A. Progress in the Treatment of Proliferative
Lupus Nephritis. Curr Opin Nephrol Hypertens 2000; 9(2):
10715.
(Reviews potential newer therapies for lupus nephritis.)
Pharmacodynamic changes include the following.
The sensitivity of the brain to the effects of some psychoactive
drugs is increased.
Tissue sensitivity to the effects of some endogenous hormones
(including growth hormone, vitamin D analogues and insulin) is
reduced.
Sensitivity to the effects of acetylcholinesterase inhibitors is
increased.
Changing drug regimens in renal insufficiency
A drug dosing regimen is a recipe for drug administration, intended to produce the desired therapeutic effect with a minimum
of unwanted effects. It is described in terms of the dose of drug,
the frequency and route of administration, and the formulation
used.
It is usual to start drug therapy using the published recommendations, generally beginning at the lower end of the recommended dose range and monitoring for a therapeutic effect. If the
desired effect does not occur, increase the dose gradually, until
Practice points
Lupus nephritis is common in patients with SLE and may be
asymptomatic
Clinical features do not predict severity on renal biopsy
Development of lupus nephritis strongly predicts renal and
patient survival
Current treatment is with cortciosteroids and an
immunosuppressive agent such as intravenous
cyclophosphamide or azathioprine
Large trials are required to confirm optimum therapy and assess
newer, less toxic treatments
MEDICINE
Jeffrey K Aronson is Clinical Reader in Clinical Pharmacology at the
University of Oxford, UK, and Honorary Consultant Physician in the Oxford
Radcliffe Hospitals Trust. He trained in general medicine and clinical
pharmacology in Glasgow and Oxford. His research interests include
adverse drug reactions, control of ion transport systems in response to
drugs and diseases, and the clinical pharmacology of cardiovascular
drugs.
103
2003 The Medicine Publishing Company Ltd
También podría gustarte
- Genetic Diseases of the KidneyDe EverandGenetic Diseases of the KidneyRichard P. LiftonAún no hay calificaciones
- Palliative Care Emergencies PDFDocumento67 páginasPalliative Care Emergencies PDFmalvindersahiAún no hay calificaciones
- Nephrolithiasis: An Overview of Kidney Stone Types and PathogenesisDocumento81 páginasNephrolithiasis: An Overview of Kidney Stone Types and PathogenesisOmar Zaman KhanAún no hay calificaciones
- What Is HyperlipidemiaDocumento9 páginasWhat Is Hyperlipidemiaichanara100% (2)
- LTF InterpretationDocumento3 páginasLTF InterpretationkiethyanAún no hay calificaciones
- Fatty Liver DiseaseDocumento19 páginasFatty Liver DiseaseMaria O'ConnorAún no hay calificaciones
- 09 - Congenital SyndromesDocumento76 páginas09 - Congenital SyndromesROHIT100% (1)
- Pediatric Hematology LectureDocumento84 páginasPediatric Hematology LectureloitaAún no hay calificaciones
- Vitamin B12 Deficiency and A Patient Case StudyDocumento36 páginasVitamin B12 Deficiency and A Patient Case Studynherm6425100% (1)
- Review of Laboratory and Diagnostic TestsDocumento41 páginasReview of Laboratory and Diagnostic TestsPutri Anggraini Rusanti100% (1)
- Geriatric Medicine Lecture UploadDocumento35 páginasGeriatric Medicine Lecture UploadDoni MarthenAún no hay calificaciones
- Dka GuidelineDocumento16 páginasDka GuidelineGhada HusseinAún no hay calificaciones
- Management of Diabetes Emergencies''Documento85 páginasManagement of Diabetes Emergencies''Princewill SeiyefaAún no hay calificaciones
- Hypertension and Hemodialysis:The Silent Treatment on the Rise!De EverandHypertension and Hemodialysis:The Silent Treatment on the Rise!Calificación: 5 de 5 estrellas5/5 (1)
- Lesions of Upper Motor Neurons and Lower Motor NeuronsDocumento9 páginasLesions of Upper Motor Neurons and Lower Motor NeuronsAdhitya Rama Jr.Aún no hay calificaciones
- Drug AbreviationDocumento14 páginasDrug AbreviationGlenn Suerte100% (1)
- PEDIA 4.1 NephrologyDocumento7 páginasPEDIA 4.1 NephrologyAngela CaguitlaAún no hay calificaciones
- Abdominal Swelling + AscitesDocumento29 páginasAbdominal Swelling + AscitesDevina CiayadiAún no hay calificaciones
- HyperkalemiaDocumento8 páginasHyperkalemiaLucesita NuñezAún no hay calificaciones
- Understanding Aging: A Resource for Living BetterDe EverandUnderstanding Aging: A Resource for Living BetterAún no hay calificaciones
- Anemia in CKD Patient On HeamodialysisDocumento32 páginasAnemia in CKD Patient On HeamodialysisEditor IJTSRDAún no hay calificaciones
- Hepatobiliary Disease With AudioDocumento46 páginasHepatobiliary Disease With Audioapi-195799092Aún no hay calificaciones
- Post Stroke ComplicationsDocumento33 páginasPost Stroke Complicationsdiijah678100% (1)
- Polycystic Kidney DiseaseDocumento15 páginasPolycystic Kidney DiseaseSabita TripathiAún no hay calificaciones
- Chap1 Introduction To Nutritional AssessmentDocumento32 páginasChap1 Introduction To Nutritional AssessmentAbdi Khalaq Ali Hashi100% (1)
- Hypertension, Cardiovascular Disease, Analgesics, and Endocrine DisordersDe EverandHypertension, Cardiovascular Disease, Analgesics, and Endocrine DisordersJack Z. YetivAún no hay calificaciones
- Request Letter For PreceptorshipDocumento2 páginasRequest Letter For PreceptorshipRon OpulenciaAún no hay calificaciones
- Metabolic Syndrome: Internal Medicine Departement MF Gmu/Sardjito Hospital YogyakartaDocumento53 páginasMetabolic Syndrome: Internal Medicine Departement MF Gmu/Sardjito Hospital YogyakartaJipeeZedAún no hay calificaciones
- Alcoholic Liver Disease HarrisonDocumento3 páginasAlcoholic Liver Disease HarrisonJesly Charlies0% (1)
- Management of Diabetes Ketoacidosis in PregnancyDocumento19 páginasManagement of Diabetes Ketoacidosis in PregnancySudhir PaulAún no hay calificaciones
- Agis Mira Dewi, S.kedDocumento35 páginasAgis Mira Dewi, S.kedAgiish EMdeAún no hay calificaciones
- Case Scenario For Different Groups PDFDocumento8 páginasCase Scenario For Different Groups PDFWallen Jey VelascoAún no hay calificaciones
- Sepsis Content Concepts MapDocumento2 páginasSepsis Content Concepts Mapghodghod1230% (1)
- History Taking FormDocumento14 páginasHistory Taking FormFebbie ArcalesAún no hay calificaciones
- Operational Guidelines Acute Malnutrition South Africa FINAL-1!8!15-2Documento70 páginasOperational Guidelines Acute Malnutrition South Africa FINAL-1!8!15-2RajabSaputra100% (2)
- Identify CVD Risk in the Office with Framingham & Non-Fasting TestsDocumento28 páginasIdentify CVD Risk in the Office with Framingham & Non-Fasting TestsJuwanto Wakimin100% (1)
- Type 1 Diabetes Mellitus Clinical Presentation - History, Physical Examination, ComplicationsDocumento6 páginasType 1 Diabetes Mellitus Clinical Presentation - History, Physical Examination, ComplicationsTrifosa Ika Septiana EryaniAún no hay calificaciones
- Disorders of Calcium and Phosphate MetabolismDocumento20 páginasDisorders of Calcium and Phosphate MetabolismAhmed Noureldin AhmedAún no hay calificaciones
- Management of Diabetes Patients in SurgeryDocumento28 páginasManagement of Diabetes Patients in Surgerylow_sernAún no hay calificaciones
- Indications, Contraindications and Monitoring of Enteral NutritionDocumento13 páginasIndications, Contraindications and Monitoring of Enteral Nutritionbocah_britpopAún no hay calificaciones
- CVDDocumento22 páginasCVDvijaymusic88100% (1)
- Esrd FinalDocumento26 páginasEsrd FinalCreighton A. BayonganAún no hay calificaciones
- Exit LetterDocumento3 páginasExit Letteresteffie21Aún no hay calificaciones
- Krissa and Drentlaw Visual Acuity The Critical Measure!Documento18 páginasKrissa and Drentlaw Visual Acuity The Critical Measure!Jolien WalravenAún no hay calificaciones
- Alcoholic Hepatitis: Ekaterine Labadze MDDocumento18 páginasAlcoholic Hepatitis: Ekaterine Labadze MDsushant jainAún no hay calificaciones
- Anemia, Bleeding, and Blood Transfusion in The Intensive Care Unit: Causes, Risks, Costs, and New StrategiesDocumento29 páginasAnemia, Bleeding, and Blood Transfusion in The Intensive Care Unit: Causes, Risks, Costs, and New StrategiesDefiita FiirdausAún no hay calificaciones
- Nutrition in ICU: Enteral NutritionDocumento24 páginasNutrition in ICU: Enteral NutritionMahenderaAún no hay calificaciones
- Fluid Resuscitation and Organ Perfusion EvaluationDocumento66 páginasFluid Resuscitation and Organ Perfusion EvaluationDewiRatnasariAún no hay calificaciones
- End-Stage Renal Disease: An Integrated ApproachDe EverandEnd-Stage Renal Disease: An Integrated ApproachWilliam J. StoneAún no hay calificaciones
- Pathology of Alcoholic Liver DiseaseDocumento7 páginasPathology of Alcoholic Liver DiseasehghAún no hay calificaciones
- DKA, HHS and hypoglycemia guidelinesDocumento30 páginasDKA, HHS and hypoglycemia guidelineskuncupcupu1368Aún no hay calificaciones
- Metabolic Syndrome GuideDocumento15 páginasMetabolic Syndrome GuideMariaEllyNobetaHutabarat100% (1)
- HyponatremiaDocumento44 páginasHyponatremiaALi TaLib ShukurAún no hay calificaciones
- Anemia: Causes, Symptoms and TreatmentDocumento2 páginasAnemia: Causes, Symptoms and TreatmentLazeh MeAún no hay calificaciones
- DyspneaDocumento29 páginasDyspneaBeNz ZodiazepinAún no hay calificaciones
- Nutritional Issues in The ICU Case FileDocumento2 páginasNutritional Issues in The ICU Case Filehttps://medical-phd.blogspot.com100% (1)
- Nephrotic Syndrome in Children: January 2013Documento7 páginasNephrotic Syndrome in Children: January 2013molenAún no hay calificaciones
- Guidelines for Iron Deficiency Diagnosis & ManagementDocumento9 páginasGuidelines for Iron Deficiency Diagnosis & ManagementJohn TorresAún no hay calificaciones
- Palliative CareDocumento4 páginasPalliative CaresobanAún no hay calificaciones
- The Immunocompromised Patient Primary ImmunodeficienciesDocumento2 páginasThe Immunocompromised Patient Primary ImmunodeficienciessobanAún no hay calificaciones
- Rome III Diagnostic Criteria FGIDsDocumento14 páginasRome III Diagnostic Criteria FGIDsPutu Reza Sandhya PratamaAún no hay calificaciones
- Vulval PainDocumento3 páginasVulval PainsobanAún no hay calificaciones
- Medicolegal Issues and STIsDocumento3 páginasMedicolegal Issues and STIssobanAún no hay calificaciones
- Gastroenterology and AnaemiaDocumento5 páginasGastroenterology and Anaemiasoban100% (1)
- Urinary Tract ObstructionDocumento3 páginasUrinary Tract ObstructionsobanAún no hay calificaciones
- Acr Omega LyDocumento3 páginasAcr Omega LysobanAún no hay calificaciones
- Antineoplastic SDocumento20 páginasAntineoplastic SsobanAún no hay calificaciones
- Antiphospholipid SyndromeDocumento4 páginasAntiphospholipid SyndromesobanAún no hay calificaciones
- Malnutrition and InfectionDocumento3 páginasMalnutrition and InfectionsobanAún no hay calificaciones
- Adolescent NutritionDocumento1 páginaAdolescent NutritionsobanAún no hay calificaciones
- What's New in Respiratory DisordersDocumento4 páginasWhat's New in Respiratory DisorderssobanAún no hay calificaciones
- Analgesic & AntimigraineDocumento12 páginasAnalgesic & AntimigraineChin ChanAún no hay calificaciones
- Autonomic & NeuromuscularDocumento20 páginasAutonomic & Neuromuscularzeina32Aún no hay calificaciones
- Contraception: What's New in ..Documento4 páginasContraception: What's New in ..sobanAún no hay calificaciones
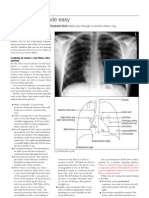
- Cara Membaca Foto Thoraks Yang BaikDocumento2 páginasCara Membaca Foto Thoraks Yang BaikIdi Nagan RayaAún no hay calificaciones
- The Wheezing InfantDocumento4 páginasThe Wheezing InfantsobanAún no hay calificaciones
- The Management of Acute Renal FailureDocumento4 páginasThe Management of Acute Renal Failuresoban100% (1)
- Non Epileptic Causes of Loss of ConsciousnessDocumento3 páginasNon Epileptic Causes of Loss of ConsciousnesssobanAún no hay calificaciones
- Disorders of PubertyDocumento2 páginasDisorders of PubertysobanAún no hay calificaciones
- Case Scenarios Nutrition Growth and DevelopmentDocumento4 páginasCase Scenarios Nutrition Growth and DevelopmentsobanAún no hay calificaciones
- Drugs That Damage The LiverDocumento5 páginasDrugs That Damage The LiversobanAún no hay calificaciones
- Neuro DegenerativeDocumento11 páginasNeuro DegenerativesobanAún no hay calificaciones
- Haemo Chroma To SisDocumento4 páginasHaemo Chroma To SissobanAún no hay calificaciones
- AppendixC NutrientChartDocumento5 páginasAppendixC NutrientChartArianne Nicole LabitoriaAún no hay calificaciones
- What Is DiabetesDocumento2 páginasWhat Is DiabetessobanAún no hay calificaciones
- Whats New in Asthma and COPDDocumento3 páginasWhats New in Asthma and COPDsobanAún no hay calificaciones
- Neuro DegenerativeDocumento11 páginasNeuro DegenerativesobanAún no hay calificaciones
- Renal Disease and PregnancyDocumento4 páginasRenal Disease and PregnancysobanAún no hay calificaciones
- The Assurance KeyDocumento2 páginasThe Assurance KeySpeech & Language Therapy in PracticeAún no hay calificaciones
- OsteomyelitisDocumento3 páginasOsteomyelitisCamille Lacson CayabyabAún no hay calificaciones
- Michael Mahoney A RetrospectiveDocumento17 páginasMichael Mahoney A RetrospectiveJonathon Bender100% (1)
- SAS #14 - Decent Work Employment - Transcultural NursingDocumento9 páginasSAS #14 - Decent Work Employment - Transcultural NursingBless O DumagoAún no hay calificaciones
- Pediatric Practice Math Problems Answer KeyDocumento3 páginasPediatric Practice Math Problems Answer KeyKaren Hutchinson50% (2)
- Bipolar Disorder Assessment and ManagementDocumento57 páginasBipolar Disorder Assessment and ManagementUniversidad de sevilla100% (1)
- Dental Trauma LectureDocumento10 páginasDental Trauma Lectureasop06Aún no hay calificaciones
- Positioning Techniques in Long-Term Care - Self-Directed Learning Package For Health Care Providers PDFDocumento41 páginasPositioning Techniques in Long-Term Care - Self-Directed Learning Package For Health Care Providers PDFAde Risaldi TangdilianAún no hay calificaciones
- Wim Hof MethodDocumento4 páginasWim Hof MethodAdiMulahasanovicAún no hay calificaciones
- Heti 2021 IPPE POSTER VOLTEIODocumento1 páginaHeti 2021 IPPE POSTER VOLTEIOAline SartiAún no hay calificaciones
- Invasive Cervical Traction Gardner Wells TongsDocumento3 páginasInvasive Cervical Traction Gardner Wells TongsErvan Hartanto ErvanAún no hay calificaciones
- Ebook Legal Abuse Syndrome PDFDocumento61 páginasEbook Legal Abuse Syndrome PDFconnecticuttruth100% (2)
- Unlicensed youth programs pose risksDocumento34 páginasUnlicensed youth programs pose risksAnthony PaulAún no hay calificaciones
- Mha Cam PDFDocumento272 páginasMha Cam PDFMax Rene Velazquez GarciaAún no hay calificaciones
- IV WorkbookDocumento51 páginasIV WorkbookAndreea Gheorghiu0% (1)
- Letter To My ParentsDocumento6 páginasLetter To My ParentsDeli Ari100% (1)
- Brief Strategic Family TherapyDocumento12 páginasBrief Strategic Family Therapymichelle458Aún no hay calificaciones
- Hospital IntroductionDocumento3 páginasHospital IntroductionSh Mati ElahiAún no hay calificaciones
- MCMI-III Interpretation GuideDocumento31 páginasMCMI-III Interpretation GuideMadalina Kit50% (6)
- Bioscalar Energy: The Healing PowerDocumento4 páginasBioscalar Energy: The Healing PowerSagarsinh RathodAún no hay calificaciones
- RCT LectureDocumento64 páginasRCT LectureAKNTAI002Aún no hay calificaciones
- Rlapse Prevent FinalDocumento5 páginasRlapse Prevent FinalBilly Mondragon100% (1)
- Narrative ReportDocumento11 páginasNarrative Reportwhatss upAún no hay calificaciones
- Hypnotism Secret 8Documento5 páginasHypnotism Secret 8parasAún no hay calificaciones
- Persuasive Speech PlanDocumento1 páginaPersuasive Speech PlanRobbin LisondraAún no hay calificaciones
- Hepatitis A VaccineDocumento5 páginasHepatitis A VaccineHåíthãm KhãtïßAún no hay calificaciones
- Pharmacokinetics of Intravenous FK027 in Healthy VolunteersDocumento10 páginasPharmacokinetics of Intravenous FK027 in Healthy VolunteersKharisma Aditya Rasyid Tf'ersAún no hay calificaciones
- Effect of Mcconnell Taping On Pain, Rom & Grip Strength in Patients With Triangular Fibrocartilage Complex InjuryDocumento9 páginasEffect of Mcconnell Taping On Pain, Rom & Grip Strength in Patients With Triangular Fibrocartilage Complex InjuryDr. Krishna N. SharmaAún no hay calificaciones
- Crackdown 15 ECDocumento6 páginasCrackdown 15 ECSadhana SentosaAún no hay calificaciones
- BSL23 PDFDocumento2 páginasBSL23 PDFTrevaAún no hay calificaciones