Documentos de Académico
Documentos de Profesional
Documentos de Cultura
Exercices Acide Base SM 12
Cargado por
khalid el yacoubiDescripción original:
Derechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Exercices Acide Base SM 12
Cargado por
khalid el yacoubiCopyright:
Formatos disponibles
1
& $"
2
)H 2 O(
3 & 5
4
)HO (aq
&3
3
6 & $ & )" * + )HSO3 (aq
7 2 / *0"
) &SO 2 , H 2 O(aq) .
2
3
)CH 3 COO (aq
8 & ") & SO (aq) .
CH 3 COOH(aq) 6
9 & "
10 &
)NH 3 (aq
7A
1 ? BC
)3 - HNO 2 (aq
............. + H +
)H 2 O(
)H 3 O (aq
> +
?0 "
............... + H +
;:
<
)1 HCOOH(aq
PO34 (aq) + H +
2 .................
4-HO (aq) + ..........
)CH 3 NH 3+ (aq
HSO (aq) / . . E
* "
) CH 3 NH 3+ (aq& $"
& : E& : 3
./
:"
7 A <
C
1 "
.
& : 3
: E
2 "
7 A + : . 6
< /H : E
3 &
4
> ; . In
;
; P ; /O 2 EN > ; HIn ; P
BP " BBT
)
H$ >HIn / In +
<
BC
1 "
H . R 2
S&
2 A )
& & 6
7
B O&T
2 *
H$ . : E
"
. & + H "
2
C& "> S & .
2 "
6
3 B O
H$ . : E
) &.
2 / *3 /"
< W& E5 E ) PO 34 (aq
/ H &* X
: E
/Y
.
): SO 2 , H 2 O(aq
)SO 2 , H 2 O(aq) + PO (aq) HSO (aq) + HPO 24 (aq
1
2
3
4
3
4
7 A
H : E : E
"Z
;.
> A + \ 7 <
: E "
*
;.
> A + \ 7 <
* /O 2 : E "
X * 5)
.
>+
:
<
C
"
6
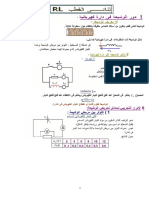
C1 = 2,50 10 2 mol / L / T
7]&> " CH 3 COOH(aq) 6& 3
2
> V1 = 20, 0mL T
2 C& ))> " (Na + (aq) + BO 2 (aq
V2 = 75, 0mL
. $
<
BC
! "
1 & ) A
"
; * > H& 2
2 : E
: /
" >
2"
H$ T
*$
3 + C2
&$ .: E
7
" pH moins " pH H & 2
2&
:
)`
_pH 7 E
C 2 = 1, 00 10 2 mol / L /
;.
<
+ < / _ ^ *
) 17,8%
) "
& )" / *0 C& & )" * + C& ). NaHSO 4 (s
) _ N O 2
1
2
3
&
H$
5 T
2
4
+ &
& )" * + )HSO (aq
>+
7 A <
BC
"
.
2
g E * 0 2 < WH
f
"
)` . V = 50m3 ; T
2
H
500g B O
i . 2
g E 0 2
&h * T
" > *$
8
2 T 7 2 * &C : 2 6
2 7 2 / * & 6 AgY
O2
)HSO (aq) / SO (aq
= 63, 0g.mol1 , p = 100%, d = 1,52
Page 1
HNO3
(M
www.chimiephysique.ma
**
Allal mahdade
1 : 2 T R : 5 2
" > 2 CHNO3 T .
2
3
4
5
6
7 * & 6 2 7 2
A7
: E
"
V = 10mL T HRZ N
7 2 T + /< $ O
Y5
50mL
)
+ 2
^R
BO ? Y Y
;
" > 2 :C2
2 $ /"
H 2 V2 ?T q
> V1 = 20mL T
^ S N
C& "
2 "
; < /
"#
"
+ < / V1 ?T2
7 * &. 6
!
*) '" & . %
>+
BC
NH3 ( g ) + H
) NH +4 ( aq ) / NH3 ( g
) CH3COOH ( ) / CH3COO ( aq
CH3COO ( aq ) + H +
) CO2 , H 2 O / HCO3 ( aq
HCO3 ( aq ) + H +
CO 2 , H 2 O
) HCO3 ( aq ) / CO32 ( aq
CO32 ( aq ) + H +
) HCO3 ( aq
) HNO3 ( ) / NO3 ( aq
NO3 ( aq ) + H +
) HNO3 (
Page 2
C&
" > . C 2 = 1mol / L
/< HO 2 .
> " X * 5 & $"
.
A 7 ! " < / > * : E 2
^ >+
A 7 . :N 2
: E
"
:" 6 $&
C& g /
2 "
V2 ?T2
2
.
& 2 V ' = 100mL ?T
$
/
. ?5
) ( aq
NH +4
) CH3COOH (
?5 7 2
&
7]&3
/ *0"
;
& " * +
7 * &6
www.chimiephysique.ma
?5
&6
& ]0
& " * +
&
& *
Allal mahdade
También podría gustarte
- Ex ReactionsDocumento7 páginasEx ReactionsTanjawa LkhawaAún no hay calificaciones
- Physics Unit12 ResumeDocumento7 páginasPhysics Unit12 ResumeQlash Mouad CrAún no hay calificaciones
- O N C B Be: Ans T TDocumento3 páginasO N C B Be: Ans T TAlicherif BenaissaAún no hay calificaciones
- 1 - Assignments in Solid State PhysicsDocumento6 páginas1 - Assignments in Solid State PhysicsjamaeeAún no hay calificaciones
- pH pH س& 3$) و: mol HO O HDocumento5 páginaspH pH س& 3$) و: mol HO O HJalal JoliloAún no hay calificaciones
- L mol C m S Cooh H C: ل !E@ا م1/ا) >1 &' (ا) E 1@' - ,Fا:G E< - 4 - &) ا آاا + I'أ "Documento4 páginasL mol C m S Cooh H C: ل !E@ا م1/ا) >1 &' (ا) E 1@' - ,Fا:G E< - 4 - &) ا آاا + I'أ "HAMADA1972Aún no hay calificaciones
- C H O (Salicylate de Méthyle) (Bouleau) Essence de Wintergreen C H O (Acide Salicylique) CH O 1 1 2 15mL 8,2g 2mLDocumento6 páginasC H O (Salicylate de Méthyle) (Bouleau) Essence de Wintergreen C H O (Acide Salicylique) CH O 1 1 2 15mL 8,2g 2mLWilliam DukeAún no hay calificaciones
- Upload 6 PDFDocumento104 páginasUpload 6 PDFRobert StefanAún no hay calificaciones
- Elghzizal'S Scribd Documents: R'-CH2-OH 67% R'-CHOH-R" 60% 5%Documento4 páginasElghzizal'S Scribd Documents: R'-CH2-OH 67% R'-CHOH-R" 60% 5%Mohamed El OuahdaniAún no hay calificaciones
- Gatgens,E. (2020). La Discrecionalidad en El Derecho Penal y Procesal Penal Costarricense, En Ciencias Penales y Derechos Humanos, Libro Homenaje Al Prof. Dr. Javier Llobet Rodríguez, San José, Costa Rica, EdiDocumento36 páginasGatgens,E. (2020). La Discrecionalidad en El Derecho Penal y Procesal Penal Costarricense, En Ciencias Penales y Derechos Humanos, Libro Homenaje Al Prof. Dr. Javier Llobet Rodríguez, San José, Costa Rica, EdiRodolfo CumbreraAún no hay calificaciones
- Chapter 11Documento5 páginasChapter 11zfjv9qgm8nAún no hay calificaciones
- Òüßbøn A@Òîjíš Nûa@Pa Yìûa@Òüü: ) Gè Jö) Æ Ëö) Üé×Ãjö) Ì ) Æ Ã Ûjš ) Üé×Ãjö) Æ Sâ SS ) Å ŞîDocumento79 páginasÒüßbøn A@Òîjíš Nûa@Pa Yìûa@Òüü: ) Gè Jö) Æ Ëö) Üé×Ãjö) Ì ) Æ Ã Ûjš ) Üé×Ãjö) Æ Sâ SS ) Å ŞîAkramAún no hay calificaciones
- Lab 7 Extraction of PB BaterryDocumento23 páginasLab 7 Extraction of PB BaterryCeka ClassicalAún no hay calificaciones
- State Service (Main) Examination - From -2020: & JK:-लH -800 MC लH: -100 MC 340562& 2: - ह (+0N)Documento16 páginasState Service (Main) Examination - From -2020: & JK:-लH -800 MC लH: -100 MC 340562& 2: - ह (+0N)Pratap LokareAún no hay calificaciones
- Open Source Development With CVSDocumento244 páginasOpen Source Development With CVSapi-3714781Aún no hay calificaciones
- TDTCDKTCNDocumento3 páginasTDTCDKTCNDũngSchildkröteAún no hay calificaciones
- KeysDocumento1 páginaKeysBảoLinhAún no hay calificaciones
- Mens Health Big Book of Exercises Issue 2013 PreviewDocumento10 páginasMens Health Big Book of Exercises Issue 2013 PreviewHelton Azevedo100% (1)
- :%&' ا K,8 6%* "F,Lا زا,MاDocumento7 páginas:%&' ا K,8 6%* "F,Lا زا,MاAbderrahim DachiAún no hay calificaciones
- :%&' ا K,8 6%* "F,Lا زا,MاDocumento7 páginas:%&' ا K,8 6%* "F,Lا زا,Mاprofpc20Aún no hay calificaciones
- VereskDocumento9 páginasVereskMehdi ShoaiAún no hay calificaciones
- Ex - Parcial Venti 23-2Documento2 páginasEx - Parcial Venti 23-2miltonAún no hay calificaciones
- نمذجة المعادلات الهيكلية باستخدام المربعات الصغرى الجزئية مثال تطبيقي باستخدام r في بحوث المحاسبة والتدقيقDocumento18 páginasنمذجة المعادلات الهيكلية باستخدام المربعات الصغرى الجزئية مثال تطبيقي باستخدام r في بحوث المحاسبة والتدقيقSaida LegouiAún no hay calificaciones
- Soluc Ep Sm835a 2023 2Documento2 páginasSoluc Ep Sm835a 2023 2miltonAún no hay calificaciones
- State Service (Main) Examination - From -2020: & JK:-लH -800 MC लH: -100 MC 340562& 2: - ह (+0N)Documento16 páginasState Service (Main) Examination - From -2020: & JK:-लH -800 MC लH: -100 MC 340562& 2: - ह (+0N)Dhiraj MohijeAún no hay calificaciones
- Earthing) or (Grounding) (Documento33 páginasEarthing) or (Grounding) (Mokr AchourAún no hay calificaciones
- Screenshot 2024-01-20 at 10.20.42 PMDocumento1 páginaScreenshot 2024-01-20 at 10.20.42 PMo.s.a.ironAún no hay calificaciones
- الوعظ الثمينDocumento136 páginasالوعظ الثمينMahmoud TarekAún no hay calificaciones
- Cour MT Civile 10Documento14 páginasCour MT Civile 10salahAún no hay calificaciones
- Lughat Ul Quran - Complete Quran Dictionary PDFDocumento39 páginasLughat Ul Quran - Complete Quran Dictionary PDFark1912Aún no hay calificaciones
- Тема 15. Показникові нерівностіDocumento9 páginasТема 15. Показникові нерівностіt8586hzqgbAún no hay calificaciones
- تقييم الترجمة - مقاربة كتارينا رايسDocumento12 páginasتقييم الترجمة - مقاربة كتارينا رايسKari KarimaAún no hay calificaciones
- Claim Form ArabicDocumento2 páginasClaim Form Arabicg1421909Aún no hay calificaciones
- Electronic FilesDocumento50 páginasElectronic FilesAsd AliAún no hay calificaciones
- Sani MahadevaDocumento9 páginasSani MahadevaBapu Rao100% (2)
- TDS Dehyton KDocumento2 páginasTDS Dehyton KCARMEN LINARES0% (1)
- Tanqiat Ul ImanDocumento364 páginasTanqiat Ul ImansunnivoiceAún no hay calificaciones
- From The Culture of Polygamy To The Right of Polygamy: January 2010Documento22 páginasFrom The Culture of Polygamy To The Right of Polygamy: January 2010zyatiAún no hay calificaciones
- CRC Electrode PotentialsDocumento10 páginasCRC Electrode PotentialsMohamedou ThiamAún no hay calificaciones
- Power PointDocumento8 páginasPower PointMaria Camila ToroAún no hay calificaciones
- Chapter 7Documento8 páginasChapter 7Boul chandra GaraiAún no hay calificaciones
- تركيب المراحيض والشطافات المختلفةDocumento65 páginasتركيب المراحيض والشطافات المختلفةمحمد عبدالرزاقAún no hay calificaciones
- Supplementary Reading MaterialDocumento6 páginasSupplementary Reading MaterialKusaal KusasiAún no hay calificaciones
- Docssecuresc63q96n0suaeoop10ft049p97gf8onfuansqd45pl3479m3tdh49co118gidmj5cu166 2Documento189 páginasDocssecuresc63q96n0suaeoop10ft049p97gf8onfuansqd45pl3479m3tdh49co118gidmj5cu166 2livingyeosangAún no hay calificaciones
- Diet For Individuals With ColitisDocumento16 páginasDiet For Individuals With Colitisjbotha01Aún no hay calificaciones
- @ A Yìûa@Òü Ü Òüßbøn A@Òîjíš Nûa@P: à ) Gè Jö) Æ Ëö) Üé×Ãjö) Ì ) Æ Ûjš ) Üé×Ãjö) Æ Sâ SS ) Å ŞîDocumento43 páginas@ A Yìûa@Òü Ü Òüßbøn A@Òîjíš Nûa@P: à ) Gè Jö) Æ Ëö) Üé×Ãjö) Ì ) Æ Ûjš ) Üé×Ãjö) Æ Sâ SS ) Å ŞîMajdi Fahmi AlkayedAún no hay calificaciones
- TIS .1227.e.2539 PDFDocumento26 páginasTIS .1227.e.2539 PDFHIMAKSHI BHATIAAún no hay calificaciones
- Properties of Solutions - 2018 Filled in Chem 1048Documento87 páginasProperties of Solutions - 2018 Filled in Chem 1048Saajid AmraAún no hay calificaciones
- IELTS Reading and Writing General Ielts-FighterDocumento159 páginasIELTS Reading and Writing General Ielts-Fighternguyen huongAún no hay calificaciones
- ﻞﻔﻄﻠﻟ ﺔﻬﺟﻮﻤﻟا ﺔﻄﺴﺒﻤﻟا ﺔﻴﻤﻠﻌﻟا صﻮﺼﻨﻟا ﺔﻤﺟﺮﺗ Translating Popular Science Texts for ChildrenDocumento14 páginasﻞﻔﻄﻠﻟ ﺔﻬﺟﻮﻤﻟا ﺔﻄﺴﺒﻤﻟا ﺔﻴﻤﻠﻌﻟا صﻮﺼﻨﻟا ﺔﻤﺟﺮﺗ Translating Popular Science Texts for ChildrenMarwa MeroAún no hay calificaciones
- Paper - 10 - MCQsDocumento8 páginasPaper - 10 - MCQsyashiperera1230Aún no hay calificaciones
- Wo PBC 12 4 CDocumento22 páginasWo PBC 12 4 Camersafi_78Aún no hay calificaciones
- Standard Electrode PotentialDocumento5 páginasStandard Electrode PotentialAli Alipor NajmiAún no hay calificaciones
- Bac Cor 08 SV1Documento5 páginasBac Cor 08 SV1Don YassineAún no hay calificaciones
- Bac Cor 08 SV1Documento5 páginasBac Cor 08 SV1Don YassineAún no hay calificaciones
- Divided States: Strategic Divisions in EU-Russia RelationsDe EverandDivided States: Strategic Divisions in EU-Russia RelationsAún no hay calificaciones
- Bhautik Evam Rasyan Vigyan: Vigyaan ki anubhutiyo ka moolik prastutikaranDe EverandBhautik Evam Rasyan Vigyan: Vigyaan ki anubhutiyo ka moolik prastutikaranAún no hay calificaciones
- Outcomes Book: Debate and Consensus after the WPA Outcomes StatementDe EverandOutcomes Book: Debate and Consensus after the WPA Outcomes StatementAún no hay calificaciones
- Devoir 2 Arsalan 2SM BIOFDocumento3 páginasDevoir 2 Arsalan 2SM BIOFkhalid el yacoubiAún no hay calificaciones
- Ensa2012 Tanger PDFDocumento4 páginasEnsa2012 Tanger PDFkhalid el yacoubiAún no hay calificaciones
- Page #1 SnapshotDocumento1 páginaPage #1 Snapshotkhalid el yacoubiAún no hay calificaciones
- تصحيح فيزياء الدورة العادية 2013 مسلك العلوم الفيزيائيةDocumento3 páginasتصحيح فيزياء الدورة العادية 2013 مسلك العلوم الفيزيائيةالغزيزال الحسن EL GHZIZAL HassaneAún no hay calificaciones