Documentos de Académico
Documentos de Profesional
Documentos de Cultura
1 s2.0 S096085241201574X Main PDF
Cargado por
Jorge Rodriguez HerreraDescripción original:
Título original
Derechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
1 s2.0 S096085241201574X Main PDF
Cargado por
Jorge Rodriguez HerreraCopyright:
Formatos disponibles
One-pot quantitative hydrolysis of lignocelluloses mediated by black liquor
Zuolin Zhu
a,b,
, Meg M. Sun
b
, Chungao Su
a
, Hongmei Zhao
a
, Xuemei Ma
a
, Zuodong Zhu
a
,
Xianlei Shi
a
, Kangfu Gu
b
a
China Fuel (Huaibei) Bioenergy Technology Development Co., Ltd., Anhui 235000, China
b
Sun Pharmaceuticals, Inc., 13718 Sorbonne Ct., San Diego, CA 92128, USA
h i g h l i g h t s
" Lignocelluloses are hydrolyzed into
small organic molecules with good
selectivity.
" Cellulose and hemicellulose into
small organic acids, lignin into small
molecular aromatics.
" Neither gasication nor black tar
formation was observed.
" Most of sulphur-chemicals in black
liquor are oxidized into sulphates.
" Oxygen-transfer between
carbohydrates and lignin may be the
possible reaction mechanism.
g r a p h i c a l a b s t r a c t
a r t i c l e i n f o
Article history:
Received 2 August 2012
Received in revised form 17 October 2012
Accepted 18 October 2012
Available online 26 October 2012
Keywords:
Lignin hydrolysis
Black liquor
Lignocellulose
Lactic acid
High octane number gasoline
a b s t r a c t
Black liquor from the kraft process facilitates quantitative biomass hydrolysis converting cellulose and
hemicellulose into organic acids such as lactic acid (50%), and lignin into small molecular aromatics,
without gasication and black tar formation. Oxygen transfer between lignin and carbohydrates may
be the mechanism. With this method, three tons of lignocellulosic biomass can potentially produce up
to one ton of lactic acid, and one ton of small molecular aromatics. This novel usage of black liquor is
environmentally viable because it is accompanied by signicant emission reduction of particulates, sulfur
and nitrogen oxides, most organic sulfur compounds and sultes of black liquor were converted into
sulfates.
2012 Elsevier Ltd. All rights reserved.
1. Introduction
On Earth Day 2012, human beings began believing that we are
approaching the Turning Point in the Fight against Global Warm-
ing. In July of 2012, Scientists conrmed that extreme weather
events are caused by man-made climate change (Peterson et al.,
2012). The survival of human beings depends on how soon we
can replace petroleum oil with regenerable resources such as bio-
mass. Fossil oil is considered as the blood of contemporary human
society, because it is the raw material of two necessities.
1. Transportation liquid fuels.
2. Fundamental organic chemicals.
In light of increased demands for energy, coupled with rapid
depletion of fossil oil resources, extensive research has been car-
ried out to identify fossil fuel alternative, especially for renewable
resources that have the following criteria: availability, economics,
acceptability, environmental and emissions, national security,
technology, and versatility. Unfortunately, all thermo-chemical
methods and bio-chemical methods researched by scientists can-
0960-8524/$ - see front matter 2012 Elsevier Ltd. All rights reserved.
http://dx.doi.org/10.1016/j.biortech.2012.10.072
Corresponding author at: China Fuel (Huaibei) Bioenergy Technology Develop-
ment Co., Ltd., Anhui 235000, China. Tel.: +86 13705613787; fax: +86
05614336155.
E-mail address: zuolinzhu@yahoo.com (Z. Zhu).
Bioresource Technology 128 (2013) 229234
Contents lists available at SciVerse ScienceDirect
Bioresource Technology
j our nal homepage: www. el sevi er . com/ l ocat e/ bi or t ech
not overcome the prot obstacle (King, 2010). Thermo-chemical
methods were developed based on coal-rening technologies and
have no product selectivity; however, biomass has much less car-
bon content and very low density. Bio-chemical methods are
developed based on grain-fermentation methods, but biomass
has much less sugar content and a crystal structure that is harder
to break down. A novel method, such as one-pot quantitative con-
version of biomass into small organic molecules with good selec-
tivity, is needed. Because this kind of technology cannot be
logically designed from coal chemical methods or fermentation
processes, they are protable methods in theory.
Black liquor is the cooking aqueous solution from the kraft pro-
cess. It contains lignin, hemicelluloses and other extracts from
wood and inorganic chemicals used in the process. Black liquor
contains more than half the energy content of the wood used for
pulp; in fact, it has been used as an energy source as far back as
the 1930s. However, the use of recovery boilers for black liquor
treatment is capital intensive and inefcient for energy recovery;
additionally, there are explosion hazards, which lead to signicant
safety concerns (Szybist et al., 2005). The recovery boilers also
cause severe pollution complications, such as particulate emis-
sions, and pollutant emission of sulfur and nitrogen compounds,
including hydrogen sulde, methyl mercaptan, dimethyl sulde,
sulfur oxides, and nitrogen oxides (The World Bank Group, 1998).
The most challenging tasks in Green Chemistry include the
successful replacement of petro-chemical feedstocks with renew-
able resources. Most organic acids are currently produced using
petro-chemical processes or from sugar fermentation. Organic
acids are important commodity products with broad applications
in many areas. For example, organic acid esters are better solvents
for paints and ink than alkane-based organic solvents because es-
ters are biodegradable with excellent dissolving power (US Pat.,
2010/0184896 A1, 2010). Esters are also better liquid fuels than
ethanol; they have higher or similar heat of combustion compared
to ethanol, they are also an easier starting material with which to
make anhydrous products as compared to ethanol. Additionally,
they are non-toxic and, compared to ethanol, are non-corrosive.
Based on current knowledge, organic acids are the most attrac-
tive products produced from biomass. Several biochemical meth-
ods known for the manufacture of organic acid products have
severe drawbacks. Because grain starch is food, processes that
use regenerable resources such as traditional glucose (from starch)
as a fermentation intermediate for organic acids are not encour-
aged. On the other hand, cost of monosaccharides made from lig-
nocellulose is high because the total sugar content (cellulose and
hemicellulose) of lignocelluloses is lower than the sugar content
of grains, and breakdown of the crystal structure of cellulose is en-
ergy intensive (Tan and MacFarlane, 2009).
In general, cellulose is the most abundant constituent of ligno-
celluloses, accounting for 3050% of biomass. Research efforts so
far have not been able to overcome the economic disadvantage
of cellulosic ethanol technology (Bozell et al., 2011). In recent
years, the transformation of lignocellulosic biomass into organic
acids has become a hot research area. One conventional method
is the disproportionation of starch and cellulose under basic condi-
tions, such as the alkaline-hydrothermal conversion method. In
this and similar methods, the loading of biomass in solvent has
to be at very low concentrations (12% wt/vol) for a higher yield
liquid product (Niemela, 1990). The yield of liquid product de-
creases to less than 30% when the loading of the biomass reaches
10% (wt/vol) (Yin et al., 2011). Another problem regarding the dis-
proportionation reaction is that it has no specicity in terms of
products; the product mixture contains more than 25 monocar-
boxylic acids, 22 dicarboxylic acids, and several cyclopentyl chem-
icals. Oxidation methods (Jin and Enomoto, 2009) have to use
strong oxidants, such as concentrated hydrogen peroxide (Zhou
et al., 2006; Jin et al., 2001) and nitric acid (Fisher and Bipp,
2005) at elevated temperatures. These processes using strong oxi-
dants have safety issues; additionally, multi-hydroxyl organic
acids such as tartaric acid, glucaric acid, gluconic acid, threonic
acid are formed. Multi-hydroxyl organic acids are difcult to iso-
late and purify; therefore, their use is limited. Unconventional
method reported recently that during the anaerobic fermentation
of biomass into carboxylate salts, reaction times were found to
be too long, with a yield of less than 16% (Aiello-Mazzarri et al.,
2006).
The method described here for converting lignocellulosic bio-
mass and food residues into simple organic acids with high speci-
city and small molecular aromatics is potentially useful towards
the goal of replacing fossil oil with renewable resources. Lactic acid
and formic acid are good candidates for this purpose; lactic acid is
the monomer of PLA and the precursor of acrylic acid, and formic
acid is the precursor of hydrogen gas. Small molecular aromatics
can be converted into substituted benzenes that are high octane
number gasoline. This technology meets all criteria of availability,
economics, acceptability, environmental emissions, national secu-
rity, technology, and versatility.
2. Methods
All lignocelluloses, such as wheat straw, pine wood chips,
Chinese date wood chips, were air-dried. They were milled to small
particles of less than 2 mm in size. The wheat straw used in this
study was composed of 40% cellulose, 22% lignin, 25% hemicellu-
lose, 13% of others such as proteins, ash, extractives, etc.; pine
wood chips was composed of 46% cellulose, 30% lignin, 17% hemi-
cellulose, 7% of others such as proteins, ash, extractives, etc.; and
Chinese date wood chip was composed of 46% cellulose, 33% lignin,
15% hemicellulose, 6% of others such as proteins, ash, extractives,
etc. All chemicals such as sodium hydroxide, hydrochloric acid, di-
methyl sulde, sodium sulfate, microcrystalline cellulose, glucose,
anthraquinone, sodium sulte, hydrogen peroxide, methylrhenium
trioxide were reagent grade or higher, and were used as received.
The following analysis and experiments used wheat straw as an
example of lignocellulosic biomass:
2.1. Cellulose content measurement
0.3 gram (M) of vacuum-dried wheat straw particles was
weighed accurately to 0.0001 gram. It was mixed thoroughly with
3 ml 72% sulfuric acid inside a centrifuge tube. The mixture was
warmed inside a 30 C water bath with occasional shaking for
one hour. Then, 84 ml distilled water was added into this mixture
and the mixture was heated at 121 C for one hour. The cooled
mixture was centrifuged, and 5 ml of the clear solution was used
for glucose analysis, after adjusting pH to 4.55.0 with calcium
hydroxide. Percent of cellulose was calculated as follows (Eq. (1)):
Cellulose% glucose concentration 0:9 0:087=M: 1
The meaning of the numbers in the equation:
0.9 ?Correct for hydration (multiply the glucose reading by 0.9
to correct for the water molecule added upon hydrolysis of the cel-
lulose polymer.
0.087 ?The total volume of assay (3 ml 72% sulfuric
acid + 84 ml distilled water).
2.2. Analysis of organic acids
The organic acids were studied using the following: HPLC, Agi-
lent 1200, column: SB-AQ, 5 lm, 4.60 250 mm; mobile phase:
0.025 M, pH 2.5 using phosphate buffer solution, ow rate:
230 Z. Zhu et al. / Bioresource Technology 128 (2013) 229234
0.6 ml/min for 010 min, 0.6 ml/min to 1.2 ml/min from 10 to
15 min, 1.2 ml/min to 0.6 ml/min from 15 to 30 min, then main-
taining at 0.6 ml/min for 45 min. A DAD detector was used at
wavelength 210 nm at a column temperature of 30 C. Sample size
was 5ll and all organic acids had retention time of less than
14 min.
Analytical standard of acids: lactic acid, succinic acid, glycolic
acid, oxalic acid, tartaric acid, gluconic acid, glyceric acid, acetic
acid, formic acid, levulinic acid, fumaric acid, malic acid, maleic
acid, 2-hydroxybutyric acid, and threonic acid.
2.3. GCMS
Agilent 7890 GC with Agilent 5975C MS; Column: DB-5,
30 m 0.25 mm 0.25 lm; Injector: 10:1 split, 250 C; Carrier
gas: helium at 1.0 ml/min; Temperature: 60 C initial, hold 5 min,
ramp at 2 C/min to 200 C, hold 5 min, ramp at 2 C/min to
280 C, hold 5 min; Detector: Agilent 5975C; transfer line temper-
ature 280 C; ion source temperature 230 C; quadruple tempera-
ture 150 C; mass range 40500 lg; ionization voltage 70 eV;
Injection volume 0.2 ll.
Dimethyl sulde in black liquor was extracted with equal vol-
ume of carbon tetrachloride, and then analyzed with GCMS.
Cyclopentenyl chemicals were characterized using GCMS.
LCMS,
Column: ACQUITY UPL BEH C18, target column temperature:
45.0 C.
Sample loop size: 10.0 ll.
Solvent name A: water;
Solvent name B: acetonitrile.
The chromatographic program: 01 min, 98% of A and 2% of B;
110 min, %A drops from 98% to 2%, meanwhile, %B increases from
2% to 98%; from 10 to 12 min, %A remains 2% and %B is 98%; from
11 to 14 min, %A increases to 98%, and %B drops to 2%. The ow rate
is 0.4 ml/min.
Mass range: 501200, Ionization mode: ES+.
NMR and elemental analysis were outsourced.
2.4. Preparation of black liquor
All black liquors were made from pine wood.
A 10 liter high pressure reactor was used, the volume usage
being 90%. All reactions were purged three times with nitrogen.
A ratio of liquor to wood of 5:1 and a ramp time of 90 min to cook-
ing temperature were used for black liquor preparation. Kraft pul-
ping was conducted for 90 min at 165 C with 18% sodium
hydroxide, 25% suldity, and 0.15% anthraquinone on chips. The
temperature was brought down to below 30 C after cooking, and
the black liquor was obtained via ltration. The solid was washed
twice with distilled water (75 ml 2), and then the washing liquid
obtained was combined with black liquor.
First, microcrystalline cellulose was chosen as starting material
for the study. All reactions were carried out in a one-liter autoclave
equipped with a mechanical stirrer. The lling volume was 90% of
the autoclave capacity. The reaction mixture was purged three
times with nitrogen before hydrolysis reactions.
2.5. Cellulose conversion to organic acids (cellulose is abbreviated as
C, and solid content of black liquor as SC. SC was 150 g and SC/
C = 1.2 is the example)
The reactions were carried out using a 1000 ml stainless steel
high pressure reactor equipped with a magnetic drive. 45 g sodium
hydroxide was dissolved in 900 ml of black liquor; 125 g cellulose
was added into the black liquor solution. The reaction mixture was
purged with nitrogen three times, with stirring, inside sealed auto-
clave. Then reaction mixture temperature was increased to 250 C.
Reaction time at 250 C was one hour for typical runs. The reaction
solution was cooled to room temperature, then acidied with
hydrochloric acid to pH 2.5. The sample was rst mixed with equal
volume of isopropanol, centrifuged, and the upper layer of the clear
solution was used for analysis.
2.6. Lignocellulose conversion to organic acids (solid content of black
liquor was 150 g, using pine wood powder as the example)
The reactions were carried out using a 1000 ml stainless steel
high pressure reactor equipped with a magnetic drive. 45 g sodium
hydroxide was dissolved in 850 ml of black liquor; 130 g pine
wood powder was added to the black liquor solution. The reaction
mixture was purged with nitrogen three times, under stirring, in-
side sealed autoclave. The reaction mixture temperature was in-
creased to 250 C. Reaction time at 250 C was 60 min for typical
runs. The reaction solution was cooled to room temperature. The
sample was rst mixed with equal volume of isopropanol, centri-
fuged, and the upper layer of the clear solution was used for
analysis.
The reaction mixture was acidied with sulfuric acid to pH 2.5
under slow stirring. Brown to bright black sticky oil formed on the
surface during acidication. This sticky oil was collected and
washed with distilled water, then dried under vacuum. The aque-
ous solution obtained after isolating aromatics was combined with
the solution from oil washing, and cooled to 0 C. Most of the so-
dium sulfate precipitated out and was collected as a solid. The
aqueous solution after salt ltration was passed through macropo-
rous resin (DA201-C). All phenols absorbed onto the resin were
washed out using hot ethanol. The careful removal of ethanol un-
der reduced pressure gave product of phenols.
2.7. Biomass (pinewood as example) hydrolysis facilitated by black
liquor (scale up)
The reactions were carried out using a 10 liter, stainless steel,
high pressure reactor equipped with a magnetic drive. 400 g so-
dium hydroxide was dissolved in 8.0 L of black liquor (contains
1.4 kg solid, 196 g organic acid (58 g formic acid, 31 g acetic acid,
26 g lactic acid, 30 g glycolic acid, 17 g succinic acid, and 34 g other
organic acids), 192 g lignin) and 1.3 kg pine wood powder was
added to the black liquor solution. The reaction mixture was
purged with nitrogen three times, under stirring, inside a sealed
autoclave. The reaction mixture temperature was increased to
250 C. The reaction time at 250 C was 60 min. When the reaction
solution was cooled to room temperature, 400 g sodium hydroxide
and 1.3 kg pine wood powder was added. Again, the reaction mix-
ture was purged with nitrogen three times, under stirring, inside a
sealed autoclave and the reaction mixture temperature was in-
creased to 250 C. The reaction time at 250 C was 60 min. The
hydrolysis was repeated one more times (3.9 kg pinewood was
used). Finally, the reaction solution was cooled to room tempera-
ture, and the total volume of the product solution was 9.13 liters.
Filtration using medium lter paper shows that no solids were
present in the nal reaction solution. The reaction mixture was
analyzed with HPLC for organic acids, GC for phenols.
The reaction mixture was acidied with sulfuric acid to pH 2.5
under slow stirring. Brown to bright black sticky oil formed on the
surface during acidication. This sticky oil was collected and
washed with distilled water, then dried under vacuum to obtain
1.34 kg. The aqueous solution obtained after isolating aromatics
was cooled to 0 C. Most of the sodium sulfate precipitated out
and was collected as a solid. The aqueous solution after salt ltra-
tion was passed through macroporous resin (DA201-C). All phenols
absorbed onto the resin were washed out using hot ethanol. The
Z. Zhu et al. / Bioresource Technology 128 (2013) 229234 231
careful removal of ethanol under reduced pressure gave the prod-
uct of phenols.
2.8. Hydrocracking of the aromatics
The reactions were carried out using a 2 liter, stainless steel,
high pressure reactor equipped with a magnetic drive. 400 g dried
aromatics and 50 g catalyst powder (1% USY catalyst and 99%
3Co8Mo/Alumina) were loaded into reactor. Then, the autoclave
was sealed and the reaction mixture was purged with nitrogen
three times. Pressurized with hydrogen, the sealed autoclave was
heated up to 360 C quickly. The pressure is about 150 atm after
the temperature reached 360 C. Stirring rate was slowly increased
from 100 rpm to 500 rpm and maintained 500 rpm for the whole
reaction period. After 2 h at 360 C, heating was stopped in order
for quick cooling to room temperature. The reaction mixture was
ltered to remove all solid materials, and the light yellow product
(245 g, 61% yield) of hydrocracking was distilled out from ltrate.
3. Results and discussion
During the optimization experiments for the biomass pretreat-
ment of cellulosic ethanol processes, we found that the black liquor
from kraft pulp can convert biomass into simple small organic
acids and small molecular aromatics with good selectivity. Unlike
alkaline hydrothermal methods that yield a mixture of products
containing more than a hundred different chemicals, our system
provides specicity for simple organic acid products. The products
formed consist exclusively of simple organic acids such as formic
acid, acetic acid, glycolic acid, lactic acid, and succinic acid. In order
to conrm the observed results, a series of experiments were car-
ried out using different kinds of biomass as starting materials, such
as pure cellulose, and lignocellulosic biomass (wood and agricul-
tural residues).
In the beginning, two kinds of black liquors were used for the
investigation. One of them was obtained from a paper company,
and the other was made in-house following the paper companys
exact procedure. A ratio of liquor to wood of 5:1, and a ramp time
of 90 min were used for black liquor preparation. Kraft pulp forma-
tion was conducted for 2 h at 165 C with 18% sodium hydroxide,
25% sulde, and 0.15% anthraquinone on chips. The average solid
content of the bought black liquor (BBL) was 15.8%, and 15.1% for
the black liquor made in-house (BLH). Dimethyl sulde in the
bought black liquor was about 14 mg/L, and 23 mg/L for the black
liquor made in-house. The total content of organic acids in the
black liquor was about 20 g/L as shown in Table 1. All data are
the average of three experiments.
The ratio of the solid content (SC) of black liquor to the cellulose
(C) was 0.5, 0.8, 1.0, 1.2, and 1.5. Subsequently, 5% sodium hydrox-
ide of the black liquor (wt/vol, for example, 5 g sodium hydroxide
was added into 100 ml of black liquor) was added to the reaction
mixture to neutralize the organic acids formed. It was observed
that the yield of simple molecular organic acids depended on the
ratios of the black liquors solid content and cellulose (Table 2).
When the ratio of black liquor solid content and cellulose was
equal or higher than 1.2 (SC of the black liquor was 150 g, and cel-
lulose used was 125 g or less, using 1 liter volume reactor), all the
cellulose was converted into simple molecular organic acids
(Fig. 1S in Supplementary Material). In addition to the ve listed
simple molecular organic acids, other organic acids were present,
although their total amounts were less than 10% for the reactions
conducted, where SC/C was equal to 1.2 or higher. For the reaction
with 125 g cellulose, the content of organic acids (analyzed using
HPLC) in the reaction mixture was higher than 160 g. By subtract-
ing the organic acids from the black liquor used, the total yield of
organic acids obtained from the 125 g of cellulose was 143 g. A
plausible reason for observing higher than 100% yield is that some
carbohydrate residue in the black liquor was also converted into
simple organic acid.
Comparing the manufacturing processes, (our process: Lignocel-
lulose ?Co-Hydrolysis ?lactic acid; Cellulosic ethanol process:
Lignocellulose ?Pretreatment ?Cellulose isolation and Purica-
tion ?Hydrolysis ?Fermentation ?ethanol. Co-Hydrolysis step
is exothermic reaction. Cost of Co-Hydrolysis step is similar to
that of Pretreatment step in cellulosic ethanol process), our meth-
od produces twice yield of the lactic acid with much less cost of en-
ergy consumption, therefore, the conversion of cellulose into
organic acids using the method we developed is much more eco-
nomical than the cellulosic ethanol process wherein 36.4 g ethanol
was the maximum yield from 125 g cellulose (Badger, 2002).
When cellulose was used in excess, such as in SC/C = 1.0, 0.8 or
0.5, unreacted cellulose was recovered after the reactions. A com-
parison study was then conducted. Using the same reaction condi-
tions (at exactly same amount of cellulose as that of SC/C = 1.2 in
case of the bought black liquor system), but using an 8% sodium
hydroxide water solution as the solvent instead of black liquor, re-
sulted in a much lower yield of simple molecular organic acids (FA
9.2, AA 2.1, LA 11, GA 3.3, SA 1.2 g/L, <24%). Most of the products
were multi-hydroxyl carboxylic acids; even cyclopentenyl chemi-
cals were observed. Black tar formation and gasication were also
observed in the reactions without the black liquor. Asphalt-like
sticky black residue is the sign of black tar formation. Higher end-
ing reaction-pressure than initial reaction-pressure, and the detec-
tion of gaseous small alkanes such as methane and ethane, were
used for the conclusion of gasication.
The results (Table 2) show that black liquor made in-house was
as effective as the bought black liquor; therefore, the remaining re-
search was conducted using the in-house black liquor. Three kinds
of lignocellulosic biomasses were used in this study. They are
wheat straw as an agricultural residue example, pine wood chips
as an example of softwood, and Chinese date wood chip as an
example of hardwood. The reaction was carried out in autoclave
reactor (1 L volume, 850 ml black liquor, and 130 g dry biomass)
with a ramp, for a 100110 min heating period. The liquid to solid
Table 1
The list of organic acids in black liquor (g/L).
Simple organic (BBL, BLH) Multi-hydroxy (BBL, BLH)
FA (formic acid: 6.3, 6.1) HGa (glucaric acid: trace, trace)
AA (acetic acid: 3.9, 3.9) HTa (tartaric acid: trace, trace)
LA (lactic acid: 3.5, 3.3) HGo (gluconic acid: trace, trace)
GA (glycolic acid: 4.3, 4.1) HTo (threonic acid: trace, trace)
SA (succinic acid: 1.8, 1.9) HGe (glyceric acid: trace, trace)
Table 2
Organic acid formed via the reaction of cellulose mediated with black liquor.
BBL BLH
SC/C Acid (g/L) SC/C Acid (g/L)
FA AA LA GA SA FA AA LA GA SA
0.5 43 21 81 25 21 0.5 41 18.7 82 19 22
0.8 41 18.6 77 23 16 0.8 41 17 80 21 15
1.0 38 17 78 18 6.2 1.0 40 16 78 19 5.6
1.2 36 16 73 18 5.3 1.2 35 15 71 18 5.1
1.5 31 14.1 61 16 4.5 1.5 29 13 62 16 5.3
For BBL, SC/C = 0.5, 30% of cellulose un-reacted; SC/C = 0.8, 12% of cellulose un-
reacted; SC/C = 1.0, 4.5% of cellulose un-reacted. For BLH, SC/C = 0.5, 29% of cellulose
un-reacted; SC/C = 0.8, 12% of cellulose un-reacted; SC/C = 1.0, 5% of cellulose un-
reacted.
232 Z. Zhu et al. / Bioresource Technology 128 (2013) 229234
ratio was 7:1 (at this ratio, the paste formed could be transferred
using a pump, but when ratio was lower than this it was very dif-
cult to handle with the pump); 5% weight sodium hydroxide was
added into the black liquor. The reaction time was 60 min and the
reaction temperature was 250 C. There was almost no solid resi-
due left after the reaction with both softwood and hardwood; how-
ever, an average of 6% solid residue was left after reaction with the
wheat straw. The pine wood chip used in 8% sodium hydroxide
(not black liquor) was the control experiment. Results are listed
in Table 3. All data listed in table are the average of three reactions.
It is easy to note the advantages of using black liquor from kraft
pulp as the mediator for converting biomass into simple organic
acid and small molecular aromatics. For wood (hardwood or soft-
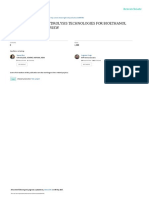
wood), a hydrolysis of 130 g biomass yielded 113 g organic acids,
40.6 g sticky oil, and 10.2 g phenols (Fig. 2S in Supplementary
Material). Elemental analysis (C 71.8%, H 7.6%, O 20.6%) and NMR
data showed that sticky oil is an aromatic product with an oxygen
content of 20.6%. Considering the oxygen content drop between
lignin in pinewood (36% oxygen) and lignin in black liquor (29%
oxygen), a quantitative conversion was observed (Fig. 1). For agri-
cultural residue such as wheat straw, about 94% conversion was
obtained. 75% of organic acid produced is simple organic acids.
The most important advantage of using black liquor is that no gas-
ication or black tar formation was observed. All organic carbons
in the lignocellulosic biomass were retained as small molecular or-
ganic products. The unreacted portion of the biomass was recov-
ered as organic material after the reaction. The control reaction
was basically a disproportionation reaction, also called an alkaline
hydrothermal conversion (HTC method), where more than 25% of
the biomass was converted into gas and black tar, and the yield
of simple organic acid products was less than 35%.
The sulfate content of black liquor was less than 0.5% when ana-
lyzed using the barium sulfate gravimetric method. This data was
used as the background. Then samples were taken from the reac-
tion mixtures of all biomasses, and each sample was divided into
two equal volumes. One of them was used for product analysis,
and the other one was acidied into pH 2.5 using hydrochloric acid,
followed by addition of 10% volume of 30% hydrogen peroxide and
1 gram of methylrhenium trioxide for catalytic oxidation. The oxi-
dation was carried out overnight with stirring under nitrogen pro-
tection, and then heated to 70 C for 60 min to decompose excess
hydrogen peroxide. Before oxidation, the solution sulfate ion con-
tent of the Chinese date wood, pinewood, and wheat straw were
4.3%, 4.2%, and 4.6% respectively. After oxidation, the values were
5.6%, 5.7%, and 5.8% respectively. It is evident that almost 85% of
suldes in the black liquor were oxidized into sulfates during the
chemical reaction of the biomass with black liquor. For dimethyl
sulde, the average concentration in the black liquor was 23 mg/
L (Chromatographic data). It was below detection limits (0.5 mg/
L) after the reaction of black liquor with cellulose and lignocellu-
losic biomass. This may be useful for reducing sulfur emissions sig-
nicantly, compared to the current boiler recovery and combustion
method of the black liquor.
Lignin is one of the main constituents of lignocelluloses. It is ac-
counted for up to 30% by weight and 40% by energy. Due to their
structural complexity, lignin received little attention relative to
cellulose with regards to valorization. It is almost commonly rec-
ognized by most scientists today that lignin can be used to make
many products, but not money. This complex natural polymer
has a molecular weight from above 3000 to 30,000 (Guerra et al.,
2006a,b). They are too big for the active cavities of the catalysts,
so they have to be depolymerized rst, and then converted into
useful molecules. Lignin depolymerization has always resulted in
gasication or/and black tar formation that causes signicant loss
of organic carbons (Zakzeski et al., 2010).
The result is a surprising one in our reported conversion of car-
bohydrates into lactic acid facilitated with black liquor. All lignin in
lignocelluloses was transformed into small molecular aromatics.
About 20% of them are phenolic compounds (about 36% is guaiacol
for pinewood), while the rest of them are the oligomers of phenyl
rings (OPR). For these oligomers of phenyl rings, more than 85%
have a molecular weight less than 400. Compared with enzymatic
mild acidolysis lignin (EMAL: C 58.2%, H 5.5%, O 36.3%) (Guerra
et al., 2006a,b), lignin from black liquor (LBL: C 63.5%, H 5.5%, S
1.6%, O 29.2%), and the small molecular aromatics (OPR: C 71.8%,
H 7.6%, O 20.6%) obtained from the biomass reaction facilitated
with black liquor, the oxygen content of OPR dropped signicantly.
Unlike all known biomass reactions, this hydrolysis reaction
(facilitated by black liquor) proceeds in a very unique way. Neither
gasication nor black tar formation was observed. No levulinic acid
was observed, but a signicant amount of formic acid was formed.
The selectivity for small organic acids and small molecular aromat-
ics does not depend on the reaction temperature, but the ratio of
lignin over cellulose plus hemicellulose [R = lignin/(cellulose +
hemicellulose)]. The temperature dependence of the process is: ob-
servable hydrolysis occurs at a temperature higher than 190 C,
and black tar formation was observed at the temperature higher
than 280 C. For R P0.5, the yield of lactic acid and phenolic com-
pounds increased for higher R. The oxygen content of the products
formed from lignin decreased signicantly; meanwhile, small or-
ganic acids have higher oxygen content than that of cellulose and
hemicellulose. All these results from our reported reaction here
suggest a novel reaction mechanism, which is the intermolecular
oxygen transfer between lignin and carbohydrates. This might be
the key step.
There are no over oxidation products (such as carbon dioxide)
and over reduction products (such as cyclohexanols) observed.
This suggests that the oxygen transfer or hydrogen transfer be-
tween lignin and carbohydrates does not proceed via free oxygen
atoms or free hydrogen atoms. Some chemicals in black liquor ex-
tract oxygen from lignin to form an active oxygen carrier. Mean-
while, lignin is depolymerized into small molecular aromatics
with reduced-oxygen content. The fragments with oxygen
Table 3
Simple molecular organic acid from biomass.
Biomass Total acid (g) FA AA LA GA SA Other acids (%)
Chinese date 108 24.4 16 46 19.8 1.8 9
Pine wood 113 25 17.5 47.5 20.1 2.0 11
Wheat straw 97 18.3 10.6 31 9.3 4.6 25
Blank 27 3.3 2.7 8.1 2.2 1.7 40
Acid concentration is g/L, acids from black liquor are included. Other acids are 2-
hydroxybutyric acid, fumaric acid, glucaric acid, tartaric acid, gluconic acid, threonic
acid, glyceric acid, and oxalic acid.
130g Pinewood
(60g cellulose + 22g Hemicellulose + 39g Lignin)
850ml Black Liquor
(21g organic acids + 20g Lignin)
113g Organic Acids
50.8g Small Aromatics
(40.6g sticky oil + 10.2g phenols)
Hydrolysis
92g Organic Acids
(>100% yield)
30.8g Small Aromatics
(99% yield)
Substract organics from black liquor
Fig. 1. Mass balance from biomass hydrolysis mediated by black liquor.
Z. Zhu et al. / Bioresource Technology 128 (2013) 229234 233
attached to benzene rings are hydrolyzed into phenols, the rest of
the lignin are converted to phenyl oligomers. The active oxygen
carrier can oxidize suldes into sulfates, and carbohydrates into or-
ganic acids. Formation of lactic acid may occur through oxygen
transfer from lignin, followed by a retro-aldol reaction reported
in literature (King, 2010).
The liquid product mixture from the biomass hydrolysis reac-
tion facilitated with black liquor can be used again as the solvent
for another biomass hydrolysis reaction. The selectivity of small or-
ganic acids did not change. Although the total yield of small molec-
ular aromatics remained the same, the yield of phenolic
compounds decreased (the absolute value is almost the same for
repeated hydrolysis), and the yield of oligomers of phenyl rings in-
creased. HPLC analysis showed that the concentration of lactic acid
is 128 g/L, formic acid is 53 g/L, glycolic acid is 59 g/L, acetic acid is
37 g/L, and succinic acid is 6.2 g/L. GC analysis showed that the
concentration of guaiacol is 2.3 g/L. The reaction mixture was acid-
ied with sulfuric acid to pH 2.5 under slow stirring. Brown to
bright black sticky oil formed on the surface during acidication.
This sticky oil was collected and washed with distilled water, then
dried under vacuum to obtain 1.43 kg. Elemental analysis and
LCMS analysis of this product showed they are similar to the aro-
matics without repeated hydrolysis (C 71.8%, H 7.9%, O 20.3%).
The aromatic product was very soluble in alcohols in room temper-
ature (higher than 30% wt/vol in methanol).
1
H-NMR showed that
the ratio of aromatic hydrogen and saturated hydrogen is about
1:4.8, very close to hydrocracking gasoline. The aqueous solution
after salt ltration was passed through macroporous resin
(DA201-C). All phenols absorbed onto the resin were washed out
using hot ethanol. The careful removal of ethanol under reduced
pressure gave 121 g product of phenols.
An additional benet of this method is that about 85% of the
sulde in black liquor is converted into sulfate during the reaction.
This is potentially very useful for signicant reductions of sulfur
emissions, especially in the paper industry. After the reuse of the
hydrolysis mixture as the solvent and mediator three times, the or-
ganic content of the mixture is about 30%. The water can be re-
placed with higher boiling point organic solvents after the
isolation of small molecular aromatics. The water distilled out con-
tains formic acid and acetic acid. These two organic acids can be
recovered from water by passing through a strong anionic ion-
exchange resin, where water can then be used as solvent again.
Hydrocracking reactions of OPR were carried out under mild
conditions. The reaction mixture was cooled down to room tem-
perature and ltered to remove all solid materials; the light yellow
product (245 g from 400 g, 61% yield) of hydrocracking was dis-
tilled out from ltrate. The GCMS analysis shows that the products
are a mixture of substituted benzenes (Fig. 3S in Supplementary
Material). Substituted benzenes are high octane number gasolines
(toluene octane number is 111 and combustion heat is 41 MJ/kg,
xylenes octane number is 117 and combustion heat is 43 MJ/kg,
ethanol combustion heat is only 26.8 MJ/kg).
4. Conclusion
Black liquor is an excellent solvent and mediator for selective
biomass hydrolysis, which results in the quantitative conversion
of carbohydrates into simple organic acid products and lignin into
small molecular aromatics. Non-free oxygen atom transfer may be
the possible reaction mechanism that was not reported up to now.
The small molecular aromatics are easily converted into substi-
tuted benzene products via hydrocracking reaction. In conclusion,
this method is the potential technology for replacing fossil oil with
lignocelluloses: organic chemicals such as small organic acids and
phenols, and liquid fuels such as substituted benzene products and
esters of organic acids.
Acknowledgement
This research was supported by grants from the key project of
the national 863 program of China (2010AA10Z404).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in
the online version, at http://dx.doi.org/10.1016/j.biortech.2012.
10.072.
References
Aiello-Mazzarri, C., Agbogbo, F.K., Holtzapple, M.T., 2006. Conversion of municipal
solid waste to carboxylic acids using a mixed culture of mesophilic
micoorganisms. Bioresour. Technol. 97, 4756.
Badger, P.C., 2002. Ethanol from cellulose: a general review. In: Janick, J., Whipkey,
A. (Eds.), Trends in New Crops and New Uses. ASHS Press, Alexandria, VA, pp.
1721.
Bozell, J.J., Black, S.K., Myers, M., Cahill, D., Paul Miller, W., Park, S., 2011. Solvent
fractionation of renewable woody feedstocks: organosolv generation of
biorenery process streams for the production of biobased chemicals.
Biomass Bioenergy 35, 41974208.
Fisher, K., Bipp, H.P., 2005. Generation of organic acids and monosaccharides by
hydrolytic and oxidative transformation of food processing residues. Bioresour.
Technol. 96, 831842.
Guerra, A., Filpponen, I., Lucia, L.A., Saquing, C., Baumberger, S., Argyropoulos, D.S.,
2006a. Toward a better understanding of the lignin isolation process from
wood. J. Agric. Food Chem. 54, 59395947.
Guerra, A., Filpponen, I., Lucia, L.A., Argyropoulos, D.S., 2006b. Comparative
evaluation of three lignin isolation protocols for various wood species. J.
Agric. Food Chem. 54, 96969705.
Jin, F.M., Kishita, A., Moriya, T., Enomoto, H., 2001. Kinetics of oxidation of food
wastes with H
2
O
2
in supercritical water. J. Supercrit. Fluids 19, 251262.
Jin, F.M., Enomoto, H., 2009. Hydrothermal conversion of biomass into value-added
products: technology that mimics nature. Bioresources 4, 704713.
King, D., 2010. The Future of Industrial Bioreneries. World Economic Forum,
Switzerland.
Niemela, K., 1990. The formation of hydroxy monocarboxylic acids and dicarboxylic
acids by alkaline thermochemical degradation of cellulose. J. Chem. Technol.
Biotechnol. 48, 1728.
Peterson, T.C., Stott, P.A., Herring, S., 2012. Explaining extreme events of 2011 from
a climate perspective. Bull. Am. Meteorol. Soc. 93, 10411067.
Szybist, J.P., Kirby, S.R., Boehman, A.L., 2005. NO
x
emissions of alternative diesel
fuels: a comparative analysis of biodiesel and FT diesel. Energy Fuels 19, 1484
1492.
Tan, S.S.Y., MacFarlane, D.R., 2009. Ionic liquids in biomass processing. Top. Curr.
Chem. 290, 311339.
The World Bank Group, 1998. Pollution Prevention and Abatement Handbook,
Washington D.C., TD897.5.P645, pp. 395400.
Bene, P., Baro, J., Schulte, H.G., US Pat., 2010/0184896 A1, 2010. Complex Esters as
Solvent for Printing Inks.
Yin, S.D., Mehrotra, A.K., Tan, Z.C., 2011. Alkaline hydrothermal conversion of
cellulose to bio-oil: inuence of alkalinity on reaction pathway change.
Bioresour. Technol. 102, 66056610.
Zakzeski, J., Bruijnincx, P.C.A., Jongerius, A.L., Weckhuysen, B.M., 2010. The catalytic
valorization of lignin for the production of renewable chemicals. Chem. Rev.
110, 35523599.
Zhou, Z., Jin, F., Enomoto, H., 2006. A continuous ow reaction system for producing
acetic acid by wet oxidation of biomass. J. Mater. Sci. 41, 15011507.
234 Z. Zhu et al. / Bioresource Technology 128 (2013) 229234
También podría gustarte
- Roger Ghanem, David Higdon, Houman Owhadi (Eds.) - Handbook of Uncertainty Quantification-Springer International Publishing (2017)Documento2035 páginasRoger Ghanem, David Higdon, Houman Owhadi (Eds.) - Handbook of Uncertainty Quantification-Springer International Publishing (2017)Jaime Andres Cerda Garrido100% (1)
- API 650 Tank DesignDocumento8 páginasAPI 650 Tank DesignJorge Rodriguez HerreraAún no hay calificaciones
- EscravosDocumento2 páginasEscravosJorge Rodriguez HerreraAún no hay calificaciones
- Posttraumatic Stress Disorder (PTSD) and War-Related StressDocumento56 páginasPosttraumatic Stress Disorder (PTSD) and War-Related Stresshiggjp3Aún no hay calificaciones
- Msi MS 7529 Rev 1.1 PDFDocumento33 páginasMsi MS 7529 Rev 1.1 PDFMisael Alves67% (3)
- Ars Lan 2010Documento7 páginasArs Lan 2010Brian Oro BeltránAún no hay calificaciones
- Project WorkDocumento27 páginasProject WorkjosephAún no hay calificaciones
- Hydrogen Production From Biomass Wastes by HydrothDocumento16 páginasHydrogen Production From Biomass Wastes by HydrothTosin OseniAún no hay calificaciones
- Levulinic Acid Production From Waste Biomass: Bioresources January 2012Documento13 páginasLevulinic Acid Production From Waste Biomass: Bioresources January 2012Fun DuniyaAún no hay calificaciones
- Thermochemical Biomass GasificationDocumento40 páginasThermochemical Biomass Gasificationjambo98Aún no hay calificaciones
- Uche MCB Project Chapter One To Three - Doc CorrectedDocumento38 páginasUche MCB Project Chapter One To Three - Doc CorrectedErhueh Kester AghoghoAún no hay calificaciones
- Env504 - Energy and Environment: BiogasDocumento15 páginasEnv504 - Energy and Environment: BiogasPapilon RougeAún no hay calificaciones
- MS-AGS32F20 Muhammad AminDocumento6 páginasMS-AGS32F20 Muhammad AminUsama HassanAún no hay calificaciones
- Anaerobic TolueneDocumento11 páginasAnaerobic TolueneEmiliano Rodriguez TellezAún no hay calificaciones
- Acid and Alkaline Pretreatment of Lignocellulosic Biomass To Produce Ethanol As Biofuel PDFDocumento8 páginasAcid and Alkaline Pretreatment of Lignocellulosic Biomass To Produce Ethanol As Biofuel PDFVincent MalayaoAún no hay calificaciones
- Acid and Alkaline Hydrolysis Technologies For Bioethanol Production: An OverviewDocumento14 páginasAcid and Alkaline Hydrolysis Technologies For Bioethanol Production: An Overvieweychezdy07Aún no hay calificaciones
- Final Year Report AfnanDocumento57 páginasFinal Year Report AfnanJr BagaporoAún no hay calificaciones
- Biological Hydrogen Production MethodsDocumento9 páginasBiological Hydrogen Production MethodsVeena mitraAún no hay calificaciones
- A Sustainable Approach For Carbon Dioxide FixationDocumento4 páginasA Sustainable Approach For Carbon Dioxide Fixation0721673895Aún no hay calificaciones
- Introduction 1Documento9 páginasIntroduction 1111 122Aún no hay calificaciones
- Hydrogen Production From Biomass Wastes by Hydrothermal GasificationDocumento15 páginasHydrogen Production From Biomass Wastes by Hydrothermal GasificationHartono PrayitnoAún no hay calificaciones
- A Mini Review On Pyrolysis and Anaerobic Digestion An OptionalDocumento10 páginasA Mini Review On Pyrolysis and Anaerobic Digestion An OptionalNDUNGUTSE JEAN MAURICEAún no hay calificaciones
- Paper BioDocumento17 páginasPaper BioI-hana D'yanaAún no hay calificaciones
- Bioenergy and SciencesDocumento7 páginasBioenergy and SciencesBuarin Meen BoorinAún no hay calificaciones
- Unit 4&5Documento28 páginasUnit 4&52k21cse093Aún no hay calificaciones
- 10 1016@j Ijhydene 2013 09 003Documento10 páginas10 1016@j Ijhydene 2013 09 003Gregorius BudiantoAún no hay calificaciones
- Bioethanol - The Fuel of Tomorrow From The Residue of TodayDocumento8 páginasBioethanol - The Fuel of Tomorrow From The Residue of TodaytvdaihaiAún no hay calificaciones
- Ionic Liquids and Deep Eutectic Solvents For Biodiesel Synthesis: A ReviewDocumento10 páginasIonic Liquids and Deep Eutectic Solvents For Biodiesel Synthesis: A ReviewMardaru AnamariaAún no hay calificaciones
- Biogas EnergyDocumento6 páginasBiogas EnergyHayan JanakatAún no hay calificaciones
- Biomass EnergyDocumento13 páginasBiomass EnergySuprava GhoshPaulAún no hay calificaciones
- Green ChemistryDocumento37 páginasGreen ChemistryDepun MohapatraAún no hay calificaciones
- Biorefinery Concept of Sugarcane Bagasse Copy-2-1Documento10 páginasBiorefinery Concept of Sugarcane Bagasse Copy-2-1geromelkAún no hay calificaciones
- Green Chemistry Application For Sustainable DevelopmentDocumento26 páginasGreen Chemistry Application For Sustainable DevelopmentAlinaCrinaCiubotariuMuresanAún no hay calificaciones
- Fermentation UthrDocumento12 páginasFermentation UthrJher OcretoAún no hay calificaciones
- Life Cycle Analysis of The Production of Biodiesel From MicroalgaeDocumento15 páginasLife Cycle Analysis of The Production of Biodiesel From MicroalgaeHema RawindranAún no hay calificaciones
- Bioresource Technology: Ke Zhang, Zhijian Pei, Donghai WangDocumento13 páginasBioresource Technology: Ke Zhang, Zhijian Pei, Donghai WangAna Sofia Rojas CarpioAún no hay calificaciones
- Answer: Research Methode Name: Haniefan Adi Kuncoro NIM: 1951180019Documento8 páginasAnswer: Research Methode Name: Haniefan Adi Kuncoro NIM: 1951180019Haniefan AdiAún no hay calificaciones
- Haniefan Adi KuncoroDocumento8 páginasHaniefan Adi KuncoroHaniefan AdiAún no hay calificaciones
- Methods For Pretreatment of Lignocellulosic Biomass For Efficient Hydrolysis and Biofuel ProductionDocumento18 páginasMethods For Pretreatment of Lignocellulosic Biomass For Efficient Hydrolysis and Biofuel ProductionFrank O'cengAún no hay calificaciones
- Tao SX HydroDocumento0 páginasTao SX HydrobiappleAún no hay calificaciones
- Energy Optimization in Fischer-Tropsch Process: Department of Chemical Engineering, Government Engineering CollegeDocumento32 páginasEnergy Optimization in Fischer-Tropsch Process: Department of Chemical Engineering, Government Engineering CollegeAdithyaAún no hay calificaciones
- Mass Balance For Biomass and Biogas Production From Organic WasteDocumento14 páginasMass Balance For Biomass and Biogas Production From Organic WastedavidchemwenoAún no hay calificaciones
- Bioresource TechnologyDocumento8 páginasBioresource TechnologyHerlin HerliansahAún no hay calificaciones
- SmolinskiHowaniec 16Documento11 páginasSmolinskiHowaniec 16Jesús Alfonso Vázquez BarragánAún no hay calificaciones
- RER Mod4@AzDOCUMENTS - inDocumento28 páginasRER Mod4@AzDOCUMENTS - inrahulAún no hay calificaciones
- Bio-Based Polymers: Irina Voevodina, Andrej KržanDocumento8 páginasBio-Based Polymers: Irina Voevodina, Andrej KržanPiero Valdivia BerroaAún no hay calificaciones
- RRL 2Documento9 páginasRRL 2Ashley Lorenz PeAún no hay calificaciones
- Process of PetrochemicalDocumento11 páginasProcess of Petrochemicallollol91Aún no hay calificaciones
- Review of Related LiteratureDocumento6 páginasReview of Related Literaturenouny234Aún no hay calificaciones
- Synthesis and Biological Application of PolylacticDocumento18 páginasSynthesis and Biological Application of PolylacticNelson Enrique Bessone MadridAún no hay calificaciones
- 04 GRAAGE Case Study BiofuelDocumento6 páginas04 GRAAGE Case Study BiofuelBojan VojvodicAún no hay calificaciones
- Hydrogen Production by Dark Fermentation - AidicDocumento6 páginasHydrogen Production by Dark Fermentation - AidicĐêmTrắngAún no hay calificaciones
- Biomass: The Ultimate Source of Bio Energy: Sushmita Mohapatra, Kasturi GadgilDocumento4 páginasBiomass: The Ultimate Source of Bio Energy: Sushmita Mohapatra, Kasturi GadgilRaghu RamAún no hay calificaciones
- Antonopoulou 2016Documento41 páginasAntonopoulou 2016Julio MoralesAún no hay calificaciones
- GOOD Pretreatment BRTL18-19Documento28 páginasGOOD Pretreatment BRTL18-19Ayush ModiAún no hay calificaciones
- Toxins: Pyrolysis of High-Ash Natural Microalgae From Water Blooms: Effects of Acid PretreatmentDocumento16 páginasToxins: Pyrolysis of High-Ash Natural Microalgae From Water Blooms: Effects of Acid PretreatmentmnjklnlAún no hay calificaciones
- 生化工程 IntroductionDocumento3 páginas生化工程 Introductiongn0033609212Aún no hay calificaciones
- Organosolv Pretreatment of Lignocellulosic Biomass For Enzymatic HydrolysisDocumento13 páginasOrganosolv Pretreatment of Lignocellulosic Biomass For Enzymatic HydrolysisDavid Alejandro GuerreroAún no hay calificaciones
- Young Innovators Program 2022: Greenhouse Gas Artificial PhotosynthesisDocumento10 páginasYoung Innovators Program 2022: Greenhouse Gas Artificial Photosynthesisneetus creationAún no hay calificaciones
- 06 Green Chemistry 06 - 10 - 2010Documento12 páginas06 Green Chemistry 06 - 10 - 2010Saurav GuptaAún no hay calificaciones
- Principles of Green Chemistry With ApplicationsDocumento20 páginasPrinciples of Green Chemistry With ApplicationsKiran BhoirAún no hay calificaciones
- Making Crude Biodiesel From Vegetable and Coconut OilDocumento7 páginasMaking Crude Biodiesel From Vegetable and Coconut Oilapi-273814635Aún no hay calificaciones
- Fungi and Lignocellulosic BiomassDe EverandFungi and Lignocellulosic BiomassChristian P KubicekAún no hay calificaciones
- Analytical Methods for Biomass Characterization and ConversionDe EverandAnalytical Methods for Biomass Characterization and ConversionAún no hay calificaciones
- Cartilla AntorchaDocumento1 páginaCartilla AntorchaJorge Rodriguez HerreraAún no hay calificaciones
- Crude Column ProiiDocumento4 páginasCrude Column ProiiJorge Rodriguez HerreraAún no hay calificaciones
- Zeus Energy SAC Peru +51949827888 Quote Number:: Pressure Relief Valve Sizing & Selection ReportDocumento5 páginasZeus Energy SAC Peru +51949827888 Quote Number:: Pressure Relief Valve Sizing & Selection ReportJorge Rodriguez HerreraAún no hay calificaciones
- Feed Heater Train: Getting Started in Steady StateDocumento12 páginasFeed Heater Train: Getting Started in Steady StateJorge Rodriguez HerreraAún no hay calificaciones
- Gasoducto DinamicoDocumento18 páginasGasoducto DinamicoJorge Rodriguez HerreraAún no hay calificaciones
- Hot Crude Oil StorageDocumento1 páginaHot Crude Oil StorageJorge Rodriguez HerreraAún no hay calificaciones
- Er V Ka 5 GBDocumento2 páginasEr V Ka 5 GBJorge Rodriguez HerreraAún no hay calificaciones
- Petro PeruDocumento3 páginasPetro PeruJorge Rodriguez HerreraAún no hay calificaciones
- Debottlenecking Options For A CDU FurnaceDocumento2 páginasDebottlenecking Options For A CDU FurnaceJorge Rodriguez HerreraAún no hay calificaciones
- Crude Summary Report: Crude: OSEBERG 2016 04 Reference: OSEBERG201604Documento3 páginasCrude Summary Report: Crude: OSEBERG 2016 04 Reference: OSEBERG201604Jorge Rodriguez HerreraAún no hay calificaciones
- 1 s2.0 S0926669007000398 Main - 12 PDFDocumento9 páginas1 s2.0 S0926669007000398 Main - 12 PDFJorge Rodriguez HerreraAún no hay calificaciones
- Degradation of Lignin in Pulp Mill Wastewaters by White-Rot Fungi On BiofilmDocumento7 páginasDegradation of Lignin in Pulp Mill Wastewaters by White-Rot Fungi On BiofilmJorge Rodriguez HerreraAún no hay calificaciones
- Yahussain@Just Edu JoDocumento4 páginasYahussain@Just Edu JoJorge Rodriguez HerreraAún no hay calificaciones
- 1 s2.0 S1226086X14001221 MainDocumento6 páginas1 s2.0 S1226086X14001221 MainJorge Rodriguez HerreraAún no hay calificaciones
- Microbiology QuestionsDocumento5 páginasMicrobiology QuestionsNaeem AminAún no hay calificaciones
- MR - Samaksh Jhalani Machinery-FinalDocumento45 páginasMR - Samaksh Jhalani Machinery-FinalSamaksh JhalaniAún no hay calificaciones
- A. Questions: Conversation Activities - TravelDocumento11 páginasA. Questions: Conversation Activities - TravelkicsirekaAún no hay calificaciones
- What Is Kpag?: Table of ContentsDocumento2 páginasWhat Is Kpag?: Table of Contentsangelito bernalAún no hay calificaciones
- HP COMPAQ D330UT-Network & InternetDocumento20 páginasHP COMPAQ D330UT-Network & Internetgebo_manAún no hay calificaciones
- ClimateDocumento38 páginasClimateCristine CaguiatAún no hay calificaciones
- Discussion 2 Module 2 - Paronda PDFDocumento1 páginaDiscussion 2 Module 2 - Paronda PDFAlvanna ParondaAún no hay calificaciones
- Balance Diet and NutritionDocumento9 páginasBalance Diet and NutritionEuniceAún no hay calificaciones
- Research Article: International Research Journal of PharmacyDocumento5 páginasResearch Article: International Research Journal of PharmacyAlfrets Marade SianiparAún no hay calificaciones
- Warm and Humid GREEN BUILDING CASE STUDYDocumento8 páginasWarm and Humid GREEN BUILDING CASE STUDYPooja PrakashAún no hay calificaciones
- 螳螂拳七長八短 - Tanglangquan Qi Chang Ba Duan - Tanglangquan's Seven Long & Eight Short - Lessons Learned in the World of Martial ArtsDocumento2 páginas螳螂拳七長八短 - Tanglangquan Qi Chang Ba Duan - Tanglangquan's Seven Long & Eight Short - Lessons Learned in the World of Martial ArtsGianfranco MuntoniAún no hay calificaciones
- Kyocera Mita KM1505 1510 1810 Series ELITEC EssentialsDocumento6 páginasKyocera Mita KM1505 1510 1810 Series ELITEC EssentialsJaime RiosAún no hay calificaciones
- Module I: Introduction To Environmental PollutionDocumento14 páginasModule I: Introduction To Environmental PollutionAman John TuduAún no hay calificaciones
- Ken Russell Revealed:: Still Images 1954 - 1957Documento4 páginasKen Russell Revealed:: Still Images 1954 - 1957Adriana ScarpinAún no hay calificaciones
- Problem Set Solution QT I I 17 DecDocumento22 páginasProblem Set Solution QT I I 17 DecPradnya Nikam bj21158Aún no hay calificaciones
- Crop Prot 2 Final Edited (Checked)Documento108 páginasCrop Prot 2 Final Edited (Checked)Cortez ReztyAún no hay calificaciones
- 2019 MIPS Quality Historic BenchmarksDocumento158 páginas2019 MIPS Quality Historic BenchmarksJoe GellatlyAún no hay calificaciones
- Plugs, Fuses and Household ElectricityDocumento4 páginasPlugs, Fuses and Household ElectricityRonald HuynhAún no hay calificaciones
- Carbon Steel Fittings DimensionsDocumento3 páginasCarbon Steel Fittings DimensionsgiorselAún no hay calificaciones
- NSC Solution F2 enDocumento8 páginasNSC Solution F2 ensaeidAún no hay calificaciones
- Rossmann Repair Training Guide - Google SlidesDocumento167 páginasRossmann Repair Training Guide - Google Slidesmirza baigAún no hay calificaciones
- Derivation of Gravity Loads PDFDocumento4 páginasDerivation of Gravity Loads PDFHenry TuganoAún no hay calificaciones
- Atomic Structure RevisionDocumento4 páginasAtomic Structure RevisioncvAún no hay calificaciones
- Carbohydrate-Related Diseases Term PaperDocumento5 páginasCarbohydrate-Related Diseases Term Paperheiress comiaAún no hay calificaciones
- Astm D6321-98-2004Documento3 páginasAstm D6321-98-2004Thyagu LingamurthyAún no hay calificaciones
- 06-Soil Fert Nutr MGTDocumento8 páginas06-Soil Fert Nutr MGTAndres LuqueAún no hay calificaciones
- Physics Assessment 1 - Lab Report: Jessica Yam Year 10 Peace MR - SlosbergDocumento19 páginasPhysics Assessment 1 - Lab Report: Jessica Yam Year 10 Peace MR - Slosbergapi-36149866550% (2)