Documentos de Académico
Documentos de Profesional
Documentos de Cultura
Dean Stark Apparatus Lab Report
Cargado por
Muhammad Naqiuddin Bin ZahidDerechos de autor
Formatos disponibles
Compartir este documento
Compartir o incrustar documentos
¿Le pareció útil este documento?
¿Este contenido es inapropiado?
Denunciar este documentoCopyright:
Formatos disponibles
Dean Stark Apparatus Lab Report
Cargado por
Muhammad Naqiuddin Bin ZahidCopyright:
Formatos disponibles
1
1.0 SUMMARY
The objectives of this saturation determination experiment are to study the procedures in
cleaning of the core samples from residual fluids and to define and determine the oil and gas
saturation of a core sample using the Dean-stark distillation-extraction method. But due to
technical and laboratory problem, we were unable to conduct this experiment. However, we
managed to do some research and finding about saturation determination, procedure in
cleaning of the core samples from residual fluids and about the Dean-stark distillation-
extraction method in order to achieve the understanding and objectives of this experiment
without conducting the experiment.
We suppose to heat the hydrocarbon solvent which is toluene to its boiling point which is 110
C. Its vapour will move upward and the rock sample becomes immerse in the toluene
vapours that begin to extract the oil and water present in the rock sample. Then the rising
vapour will be condense in condenser and collected in the graduated tube.
Since toluene is completely miscible with the extracted oil, the condensed liquid in the
graduated tube will consist of two liquid phases which are water and mixed hydrocarbon
phase containing toluene and oil from the rock sample. Due to higher density, the water phase
will settles at the bottom of the graduated tube while the solvent overflow and drips back over
the rocks sample. This process should be continuing until no more water is collect in the
receiving tube.
2
2.0 INTRODUCTION
When the core arrives in the laboratory, plugs are usually drilled 20 to 30 cm apart
throughout the reservoir interval. All these plugs are analyzed with respect to porosity,
permeability saturation and lithology. Fluid saturation can be determined by a few methods
which include injection of solvent, centrifuges flushing, gas driven solvent extraction and
Dean-stark distillation-extraction.
The Dean-stark distillation-extraction is appropriate for plug samples and for rotary sidewall
cores. This method of determination fluids saturation depends upon the distillation of water
fraction and solvent extraction of oil fraction from the sample. Besides, this method provides
a direct determination of water content. The oil and water are extracted by dripping a solvent,
usually toluene or a mixture of acetone and chloroform aver the plug samples. In this method,
water and solvent are vaporized, re-condensed in cooled tube in on the top of apparatus and
water is collected in calibrated chamber.
The set up basically consist of a longneck round-bottom flask that contains a suitable
hydrocarbon solvent such as toluene, a heating element or electric heater to boil the solvent, a
condenser and a graduated tube receiver to measure the volume of extracted fluids.
3.0 OBJECTIVES
1. To study the procedure in cleaning of the core samples from residual fluids.
2. To define and determine the oil and gas and water saturation of a core sample using
Dean-stark distillation - extraction method.
3
4.0 THEORY
Fluid saturation can be determined by a few methods which includes injection of solvent,
centrifuges flushing, gas friven solvent extraction and Dean-stark distillation-extraction. The
Dean-stark Distillation-extraction method of determining fluids saturation depends upon the
distillation of water fraction and solvent extraction of oil fraction from sample. The Dean-
Stark method provides a direct determination of water content. The oil and water are
extracted by dripping a solvent, usually toluene or a mixture of acetone and chloroforms over
the plug samples. In this method, water and solvent are vaporized, re-condensed in a cooled
tube in on the top of apparatus and water is collected in calibrated chamber. The Dean and
Stark procedure can be used to measure water content of a diverse range of samples, and has
been extensively used in industrial laboratories to measure water in petroleum oils. The oil
content is calculated from weight difference and therefore it is important that no sand grains
be lost from the core during the analysis, as this would result in erroneously high calculated
residual oil saturation.
The principle of operation is straightforward. When the core to be analyzed is weighed, the
resulting measurement will consist of the weight of the sand grains, as well as the oil and
water present in the pore space. The sample is then placed within a tear in the apparatus, and
this unit is suspended above a flask containing a solvent such as toluene. Whatever the
solvent, it must have a boiling point higher than water and be both immiscible with and
lighter than water. The dripping solvent mixes with oil from the sample, and both the solvent
and oil are returned to the solvent flask. The process continues until the sample is raised to
the boiling point of water. When it does, the water vaporizes, rises in the condensing tube
until it is condensed, and falls back into the calibrated tube. Because it is heavier than the
solvent, it collects at the bottom of the tube, where its volume can be measured. When
successive readings indicate no additional water recovery has occurred, we know all water
has been removed from the sample, and the water volume is recorded for further calculations.
5.0 APPARATUS
1. Dean-stark apparatus
2. Rock sample(core plug)
3. Solvent
4
6.0 PROCEDURE
1. Weigh a clean, dry thimble. Use tongs to handle the thimble.
2. Place the cylindrical core plug inside the thimble, and then quickly weigh the thimble and
sample.
3. Fill the extraction flask two-thirds full with toluene. Place the thimble with sample into the
long neck flask.
4. Tighten the ground joint fittings, but do not apply any lubricant for creating tighter joints.
Start circulating cold water in the condenser.
5. Turn on the heating jacket or plate and adjust the rate of boiling so that the reflux from the
condenser is a few drops of solvent per second. The water circulation rate should be adjusted
so that excessive cooling does not prevent the condenser solvent from reaching the core
sample.
6. Continue the extraction until the solvent is clear. Change solvent if necessary.
7. Read the volume of collected water in the graduated tube. Turn off the heater and cooling
water and place the sample into the oven (from 105 to 120 ), until the sample weight
does not change. The dried sample should be stored in desiccators.
8. Obtain the weight of the thimble and the dry core.
9. Calculate the loss in weight WL, of the core sample due to the removal of oil and water.
10. Measure the density of a separate sample of the oil.
11. Calculate the oil, water and gas saturations after the pore volume Vp of the sample is
determined (O.Torsaeter, 2000).
5
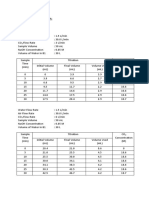
7.0 RESULTS
Sample No:
Porosity, :
W
org
g
W
dry
G
w
g/cm
3
o
g/cm
3
V
w
cm
3
W
o
G
V
o
cm
3
V
p
cm
3
S
o
S
w
S
g
Where
W
org
: Weight of original saturated sample
W
dry
: Weight of desaturated and dry sample
Equations:
W
L
= W
org
- W
dry
W
o
= W
L
- W
w
V
b
= (D/2)
2
L
V
p
= V
b
Where D and L are diameter and length of the core sample, respectively.
6
8.0 DISCUSSION
The main objective of this experiment is to determine the oil, water and gas saturation of a
core sample. Saturation is the measure of how much porosity of porous medium been
occupied by fluid. Fluid saturation is defined as the ratio of the volume of fluid in a given
core sample to the pore volume of the sample.
The experiment need to be carried in such orders as first weighed a clean, dry thimble and
then place the cylindrical core plug inside the thimble, and then quickly weigh the thimble
and sample. Next filled the extraction flask two-thirds full with toluene and placed the
thimble with sample into the long neck flask. After that, start circulating cold water in the
condenser Turn on the heating jacket or plate and adjust the rate of boiling so that the
reflux from the condenser is a few drops of solvent per second. Continued the extraction until
the solvent is clear. By the time it finish, Turn off the heater and cooling water and place
the sample into the oven (from 105
0
C to 120
0
C), until the sample weight does not change
meanwhile the dried sample should be stored in a desiccators. Then, the weight of the thimble
and the dry core is obtained in order to calculate the loss in weight WL, of the core sample
due to the removal of oil and water. Measure the density of a separate sample of the oil and
last calculate the oil and water saturation.
However, due to unavoidable technical problems we are unable to carry out this experiment
thoroughly. For the information, as we know it is important to consider the saturation change
occurring in the core from in-situ to surface conditions. Such as the condition, suppose a core
is being recovered while drilling a well with water-based drilling mud. Water from the
drilling mud will enter the rock expulsing oil. The result as the core is lifted, the reduction in
pressure will cause the oil to release gas and this will expand expulsing oil and water out of
the rock.
7
9.0 CONCLUSION
The main objective of this experiment is to determine the oil, water and gas saturation of a
core sample using the Dean-stark distillation. Through this experiment we can also study the
procedures in cleaning of the core samples from residual fluids. But due to technical and
laboratory problem, we were unable to conduct this experiment. Saturation is the important
parameter that we should know to estimate how much fluids occupied in the pore space. The
fluids are oil, water or gas. As we know it is important to consider the saturation change
occurring in the core from in-situ to surface conditions. Such as the condition, suppose a core
is being recovered while drilling a well with water-based drilling mud. Water from the
drilling mud will enter the rock expulsing oil. The result as the core is lifted, the reduction in
pressure will cause the oil to release gas and this will expand expulsing oil and water out of
the rock.
10.0 REFERENCES
1. Dean-Stark apparatus. (2013, March 15). Retrieved May 19, 2013, from Dean-Stark
apparatus: http://en.wikipedia.org/wiki/Dean-Stark_apparatus
2. O.Torsaeter, M. A. (2000, August). Experimental Reservoir Engineering Laboratory
Workbook. Retrieved May 19, 2013, from Experimental Reservoir Engineering
Laboratory Workbook: http://www.ipt.ntnu.no/~oletor/kompendium4015.pdf
3. http://www.ipt.ntnu.no/~oletor/kompendium4015.pdf
8
11.0 APPENDICES
Figure 1: Modified Dean and Stark Extraction apparatus for determining Toluene insoluble in
phosphorus (Dean-Stark apparatus, 2013).
9
Figure 2: Dean Stark apparatus (Dean-Stark apparatus, 2013).
Figure 3: Dean-Stark apparatus (Dean-Stark apparatus, 2013)
1: Stirrer bar/anti-bumping granules
2: Still pot
3: Fractionating column
4: Thermometer/Boiling point temperature
5: Condenser
6: Cooling water in
7: Cooling water out
8: Burette
9: Tap
10: Collection vessel
También podría gustarte
- Dean Stark Apparatus Lab ReportDocumento9 páginasDean Stark Apparatus Lab ReportSouvik Paul0% (2)
- Dean StarkDocumento15 páginasDean Starkfaizuanismail100% (1)
- Determining Fluid Saturation in Core SamplesDocumento6 páginasDetermining Fluid Saturation in Core SamplesMohammef MohammedAún no hay calificaciones
- Core Cleaning Lab ReportDocumento19 páginasCore Cleaning Lab ReportJombaca Berubah0% (2)
- Differential Liberation - LabDocumento12 páginasDifferential Liberation - LabAhmed AmirAún no hay calificaciones
- Regional Government of KurdistanDocumento16 páginasRegional Government of KurdistanMohammed MohammedAún no hay calificaciones
- Differential Liberation Test Experiment No.2.: University of Zakho Collage of Engineering Petroleum DepartmentDocumento15 páginasDifferential Liberation Test Experiment No.2.: University of Zakho Collage of Engineering Petroleum DepartmentSoma BerwariAún no hay calificaciones
- Reservoir Lab Manual 2015-16Documento39 páginasReservoir Lab Manual 2015-16Abhinav Sharma67% (3)
- Reservoir Rock Properties Lab.: Capillary PressureDocumento6 páginasReservoir Rock Properties Lab.: Capillary PressureHeven HassanAún no hay calificaciones
- Laboratory Analysis For Reservoir FluidsDocumento53 páginasLaboratory Analysis For Reservoir FluidsNizar Ali100% (1)
- Reservoir Lab Manual 2016-17Documento37 páginasReservoir Lab Manual 2016-17Amit Verma100% (1)
- Residue CarbonDocumento7 páginasResidue CarbonAram IbrahimAún no hay calificaciones
- CCE Experiment for Determining Bubble Point PressureDocumento6 páginasCCE Experiment for Determining Bubble Point PressureNasih NooriAún no hay calificaciones
- Sedimentation Studies Apparatus DesignDocumento7 páginasSedimentation Studies Apparatus Designgrkhari1100% (2)
- Heriot Watt University Reservoir SimulationDocumento485 páginasHeriot Watt University Reservoir SimulationBalen M. KhdirAún no hay calificaciones
- MT4 Lab FinalDocumento19 páginasMT4 Lab FinalAmelia MaharajAún no hay calificaciones
- Core Plugging: University of Karbala College of Engineering Reservoir Engineering Lab Third StageDocumento9 páginasCore Plugging: University of Karbala College of Engineering Reservoir Engineering Lab Third StageMohamed Moder100% (1)
- Reservoir Engineering Lab REPORT SESSION/SEM: 20212022/1: Experiment No. Title Section Group No. Group MembersDocumento11 páginasReservoir Engineering Lab REPORT SESSION/SEM: 20212022/1: Experiment No. Title Section Group No. Group MembersDHANASEELAN A/L V G PRAGASAM A19ET0053Aún no hay calificaciones
- 5 34-10-17 PVT Analysis of Bottom Hole SampleDocumento21 páginas5 34-10-17 PVT Analysis of Bottom Hole SampleswpuxiaofanAún no hay calificaciones
- Siti Nurina Adlina Binti Roslan 2017466054Documento27 páginasSiti Nurina Adlina Binti Roslan 2017466054Amira100% (2)
- ASTM DistillationDocumento23 páginasASTM Distillationtri_bobAún no hay calificaciones
- Reservoir Fluids Properties BookDocumento219 páginasReservoir Fluids Properties Bookkexadex2100% (5)
- PVT DaneshDocumento202 páginasPVT Daneshegv2000Aún no hay calificaciones
- Measuring Liquid ViscosityDocumento11 páginasMeasuring Liquid ViscositySiyar SaleemAún no hay calificaciones
- Methanol-Water VLE StudyDocumento4 páginasMethanol-Water VLE StudyAmeerul AhwazAún no hay calificaciones
- 010 Surface SamplingDocumento13 páginas010 Surface SamplingfarajAún no hay calificaciones
- API Gravity - Wikipedia, The Free Encyclopedia PDFDocumento3 páginasAPI Gravity - Wikipedia, The Free Encyclopedia PDFRamu NallathambiAún no hay calificaciones
- Heat of Solution ReportDocumento29 páginasHeat of Solution ReportFavourAún no hay calificaciones
- Investigation of Liquid-Solid and Gas-Solid Fluidized BedDocumento18 páginasInvestigation of Liquid-Solid and Gas-Solid Fluidized Bedmahbub1332100% (1)
- ELA Heat of SolutionDocumento15 páginasELA Heat of SolutionJimAún no hay calificaciones
- Ee - Lab ReportDocumento36 páginasEe - Lab ReportNoshaba Noreen75% (4)
- Reservoir EngineeringDocumento914 páginasReservoir EngineeringYerbolSultanbayevAún no hay calificaciones
- Well Testing SLBDocumento25 páginasWell Testing SLBfergot2010Aún no hay calificaciones
- University Petroleum Testing Lab ManualDocumento40 páginasUniversity Petroleum Testing Lab ManualVishesh Sharma100% (1)
- Lab 10 - Measurement of The Oil, Water and Solid Contents of Drilling Mud Sample Using Retort Kit (10 & 50 ML) .Documento12 páginasLab 10 - Measurement of The Oil, Water and Solid Contents of Drilling Mud Sample Using Retort Kit (10 & 50 ML) .Sunny BbaAún no hay calificaciones
- CLB11003 - Exp 4Documento6 páginasCLB11003 - Exp 4Nur DiyanahAún no hay calificaciones
- Reservior Petrophysics LabDocumento55 páginasReservior Petrophysics Labhussein alsaedeAún no hay calificaciones
- Shawket Exam Questions Dec 06Documento68 páginasShawket Exam Questions Dec 06weldsvAún no hay calificaciones
- Boys Gas CalorimeterDocumento11 páginasBoys Gas CalorimeterPraneeth Weerathunga100% (3)
- Sajad FalahDocumento12 páginasSajad FalahSajad FalahAún no hay calificaciones
- List of Methods For Well TestingDocumento6 páginasList of Methods For Well TestingRizwan FaridAún no hay calificaciones
- Gas Absorption Lab ReportDocumento3 páginasGas Absorption Lab ReportNur Shaffikha Azmi100% (1)
- CPB30503 Petrochemicals & Petroleum Refining Technology Experiment 4: Determination of Reid Vapor Pressure Full Lab ReportDocumento11 páginasCPB30503 Petrochemicals & Petroleum Refining Technology Experiment 4: Determination of Reid Vapor Pressure Full Lab ReportSiti Hajar Mohamed100% (2)
- Fulmar Field Oil Case StudyDocumento31 páginasFulmar Field Oil Case StudyopeAún no hay calificaciones
- Modelling CO2 EOR and optimizing recovery using GEMDocumento66 páginasModelling CO2 EOR and optimizing recovery using GEMjimmymorelosAún no hay calificaciones
- Center of PressureDocumento10 páginasCenter of PressureMuhammad Adli Amin Mohd AmzukiAún no hay calificaciones
- Optimized Solids Suspension: Achieving Uniform Dispersion is Critical to Product QualityDocumento7 páginasOptimized Solids Suspension: Achieving Uniform Dispersion is Critical to Product QualitymichsantosAún no hay calificaciones
- Absorption in Packed Bed Lab ManualDocumento5 páginasAbsorption in Packed Bed Lab ManualAshish Verma100% (1)
- Mud Densities Exp NewDocumento8 páginasMud Densities Exp NewHafizszul FeyzulAún no hay calificaciones
- Lab. Surface TensionDocumento8 páginasLab. Surface Tensionitto35Aún no hay calificaciones
- The Five Reservoir FluidsDocumento18 páginasThe Five Reservoir FluidsFanny BalamAún no hay calificaciones
- PVT Laboratory Procedures and ReportDocumento9 páginasPVT Laboratory Procedures and ReportOdinaka Ody MadukaAún no hay calificaciones
- 1.1 Chemical Composition and Physical Property of Reservoir FluidDocumento23 páginas1.1 Chemical Composition and Physical Property of Reservoir FluidJames Freeman100% (1)
- Packed Distillation Column ExperimentDocumento20 páginasPacked Distillation Column ExperimentChan Chun ChenAún no hay calificaciones
- 26 Properties of Reservoir RockDocumento33 páginas26 Properties of Reservoir RockWahyudi Mufti100% (1)
- Thermal Properties and Temperature-Related Behavior of Rock/Fluid SystemsDe EverandThermal Properties and Temperature-Related Behavior of Rock/Fluid SystemsAún no hay calificaciones
- Cleaning and Saturation DeterminationDocumento7 páginasCleaning and Saturation DeterminationSaroo MusicAún no hay calificaciones
- Karakteristik Batuan Reservoir (Saturasi)Documento6 páginasKarakteristik Batuan Reservoir (Saturasi)Debbie NovalinaAún no hay calificaciones
- RESERVOIR ROCK AND FLUID CHARACTERIZATIONDocumento14 páginasRESERVOIR ROCK AND FLUID CHARACTERIZATIONWilman FloresAún no hay calificaciones
- Koya University Faculty of Engineering School of Petroleum and Chemical Fluid Mechanic LabDocumento19 páginasKoya University Faculty of Engineering School of Petroleum and Chemical Fluid Mechanic LabBakomora Evans WilliamsAún no hay calificaciones
- Lunar PhasesDocumento15 páginasLunar Phasesapi-262604908Aún no hay calificaciones
- Thermodynamics Tutorial MaesoDocumento58 páginasThermodynamics Tutorial MaesoCalvin LabialAún no hay calificaciones
- Organic ChemistryDocumento2476 páginasOrganic Chemistrytilakmirle75% (4)
- Ancient Method-Black GoldDocumento2 páginasAncient Method-Black GoldJoelLadjoAún no hay calificaciones
- TUTORIAL 5 - Modeling Radiation and Natural Convection - 12 Juni 2014Documento50 páginasTUTORIAL 5 - Modeling Radiation and Natural Convection - 12 Juni 2014Nadia HandayaniAún no hay calificaciones
- Multiple choice questions on mechanics, heat and thermodynamicsDocumento21 páginasMultiple choice questions on mechanics, heat and thermodynamicsGodwinAún no hay calificaciones
- Power Consuption and Distribution in LPUDocumento14 páginasPower Consuption and Distribution in LPUAnkit ShobhawatAún no hay calificaciones
- Solar Energy-Technology & ApplicationsDocumento68 páginasSolar Energy-Technology & Applicationssmartman35Aún no hay calificaciones
- Smart Cities in India The Road AheadDocumento176 páginasSmart Cities in India The Road AheadShaun GeorgeAún no hay calificaciones
- Revision (UNIT 4 & 5)Documento10 páginasRevision (UNIT 4 & 5)WKQAún no hay calificaciones
- IDU ESSAY KarthikeyaDocumento4 páginasIDU ESSAY Karthikeyakarthikeya yarlagaddaAún no hay calificaciones
- Question and Answers OscillationDocumento22 páginasQuestion and Answers OscillationVishal BhanawatAún no hay calificaciones
- By Dr. NurhayatiDocumento82 páginasBy Dr. NurhayatiRohimatul KhodijahAún no hay calificaciones
- How Wind Energy Gets to You: A Case Study of Tamil Nadu and MaharashtraDocumento10 páginasHow Wind Energy Gets to You: A Case Study of Tamil Nadu and MaharashtraChinmaya MohantyAún no hay calificaciones
- Wegmans Case Study2Documento4 páginasWegmans Case Study2marmah_hadi100% (1)
- Uji MarshallDocumento10 páginasUji MarshallNovo EkaAún no hay calificaciones
- Chapter 1 Theories and MovementsDocumento4 páginasChapter 1 Theories and MovementsJamie Ann ManalastasAún no hay calificaciones
- DOM QP ScribdDocumento5 páginasDOM QP ScribdvsanthanamAún no hay calificaciones
- Worksheet - 2 (Gas Laws, Density, Molar Mass)Documento4 páginasWorksheet - 2 (Gas Laws, Density, Molar Mass)Jose Ruben SortoAún no hay calificaciones
- Water Adsorption Ion On Mica SurfaceDocumento13 páginasWater Adsorption Ion On Mica SurfaceRajdeep GhoshAún no hay calificaciones
- Lecture No. 3 Chemical Properties of SoilsDocumento63 páginasLecture No. 3 Chemical Properties of SoilsMark Robert Catolos100% (1)
- Lecture 345Documento5 páginasLecture 345Thyrone Jay D RamirezAún no hay calificaciones
- Ground Improvement PPT ISquareRDocumento19 páginasGround Improvement PPT ISquareRsamAún no hay calificaciones
- Course OutlineDocumento3 páginasCourse OutlinehekiAún no hay calificaciones
- Site Analysis ExampleDocumento10 páginasSite Analysis ExampleTalisa Dwiyani0% (1)
- AS Q1 w1-w2 Physical ScienceDocumento2 páginasAS Q1 w1-w2 Physical Scienceemmah adiong50% (2)
- Structural and Thermal Analysis of Magnesium Based Brake Friction MaterialDocumento13 páginasStructural and Thermal Analysis of Magnesium Based Brake Friction MaterialIJRASETPublicationsAún no hay calificaciones
- Dielektrik, Ohmik Dan IrDocumento4 páginasDielektrik, Ohmik Dan IrAswin PrasetiyoAún no hay calificaciones
- Electrical EnergyDocumento28 páginasElectrical Energykyn mairen rodriguezAún no hay calificaciones
- Importance of Biosphere Reserves in IndiaDocumento12 páginasImportance of Biosphere Reserves in IndiaRachana NarayanAún no hay calificaciones